Surface tension and surface energy are measurements of unit forces that compose a material. Due to these intermolecular forces, a liquid surface is always being pulled inward. If one is to stretch the surface, work must be done to overcome the intermolecular forces. The tension on the surface of a liquid and therefore the quantity of work required to stretch that surface is measured: and these measurements correspond to the surface tension and the surface energy. The main difference between surface tension and surface energy is that surface tension measures the force per unit length of the surface whereas surface energy measures the amount of work that needs to be done per unit area in order to stretch it.
Surface Tension
Consider a sample of liquid in a container. The liquid is held together by the cohesive intermolecular forces between the molecules that make up the liquid. A molecule inside the container is being pulled in all directions by the other molecules that surround it. However, if you consider molecules at the top surface, the molecules below them are still pulling them down but there are no liquid molecules above them to pull them upward. This means that there is a net downward force on these molecules. As a result, the entire surface of the liquid is being pulled inward. It is this inward force on the surfaces that causes liquids to form roughly spherical droplets when they are free.
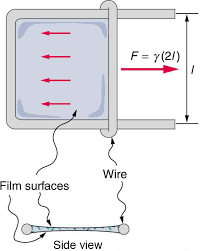
The surface of the liquid acts like a membrane underneath tension. Surface tension\gamma can be quantified as the force F acting per unit length l of the surface:\gamma =\frac
Here, the force F acts parallel to the liquid surface and is that the length over which the force acts. For example, simply imagine pulling a film of liquid toward you and then stretching it.
Surface Energy
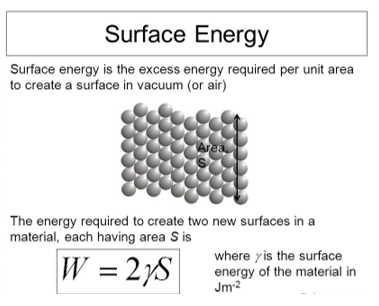
Due to the intermolecular forces between molecules, work needs to be done on a surface in order to stretch it. The surface gains an amount of energy adequate to the work that was done on the surface. Surface energy refers to the quantity of energy needed per unit space to stretch it. For the film of liquid which is mentioned above, suppose for a second the membrane is pulled through a great distance\Delta x. Then, the work is done isF\Delta x. The increase in the surface area is given by 2L\Delta x. So, the surface energy E is given by:
E=\frac=\frac=\gamma
Difference between Surface Tension and Surface Energy
What it Measures:
Surface Tension measures the force which is applied parallel to a surface which is normally applied per unit length.
Surface energy measures the energy needed per unit area to form a brand new surface.