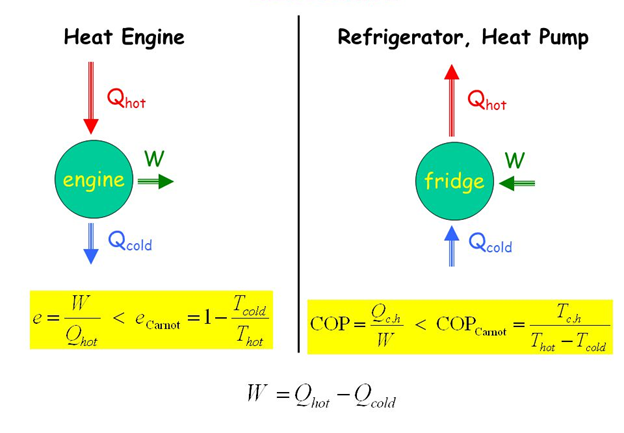
In the engineering and thermodynamics heat engine is a system for converting the chemical and thermal energy to the mechanical energy which can be used for mechanical work. This is done by bringing the substances from the state of the high temperature to the state of lower temperature. The heat source causes the generation of the thermal energy that brings the substance to the state of the high temperature. The working substance causes the generation of the work in the body of the engine while causing the transfer of the heat to the cooler sink until it is reached to a lower temperature state.
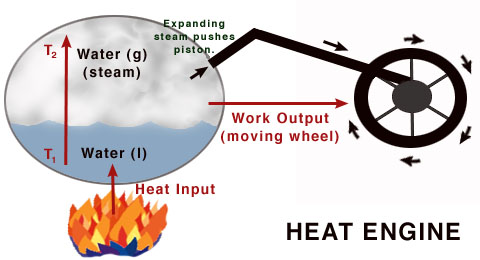
Specifications of Heat Engine
Generally, the heat engine causes the conversion of work to mechanical energy. The heat engines are distinguished from the other types of the engines because the efficiency of the heat engines is fundamentally limited by the Carnot’s theorem. Although this limitation of efficiency can be the drawback, its most significant advantage is the easy conversion of the most forms of the energy to the heat by some processes such as exothermic reactions, resistance, dissipation, friction, and absorption of energetic particles or light. As the heat source, for supplying the thermal energy to the heat engine, it can be powered by any kind of energy, so the heat engine has a wide range of applicability due to its versatile nature.
Refrigerator
The refrigerators and air conditioners are designed for cooling the things in the warmer environment. With the heat pumps, the work input is required for transferring the heat from cold to hot, and this is a much expensive approach. It works on the principle that when the two things have the different temperatures, that are touching each other or are near each other, then a balance is created in the temperature of both things are the hot substances are cooled, and the temperature of the cool substances is increased. This happens following the second law of thermodynamics.
Working of the Refrigerator
The quality of the refrigerators is judged by the amount of heat transfer. Typically, in the refrigerators, the thermodynamic system is a working fluid known as a refrigerant that is circulated by the loop. There is an alternate increase in the temperature and internal energy of the refrigerant, followed by the lowering caused by the devices in the loop. The working refrigerant is colder than the air of the refrigerator at the one point and is hotter as compared to the surroundings at another point.