The zeroth law of thermodynamics is one of the important laws of thermodynamics and it states that if the two systems are in thermal equilibrium with the third system, then these systems are in thermal equilibrium with each other.
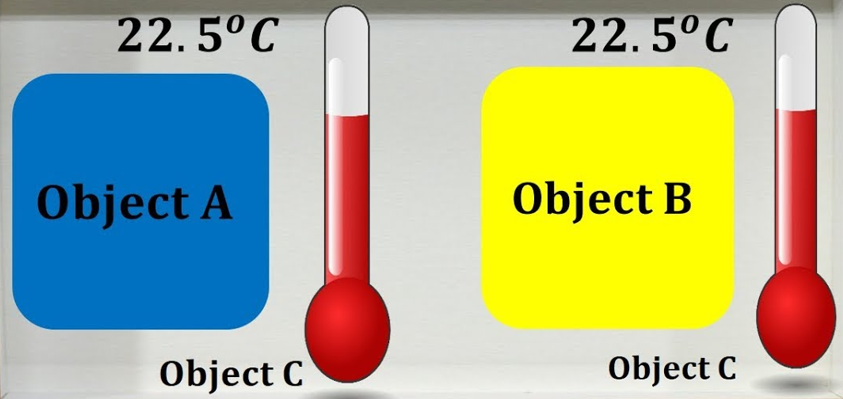
Heat Transfer Between the Objects
In thermodynamics, the relationship is studied between temperature, heat, work, and energy. Most generally, the term equilibrium is referred to as the balanced state, in which there will be no change over time. Thermal equilibrium is a situation where the heat transfer can be observed between the two objects so that these systems remain at a constant temperature over time.
Applications of Zeroth Law of Thermodynamics
The various applications of the zeroth law of thermodynamics can be seen in everyday situations. The thermometer is the most well-known and in action example of the zeroth law of thermodynamics. For example, if the thermostat in the room, gives the temperature reading of the 67-degree Fahrenheit, then it means that this thermostat is in thermal equilibrium with the room. However, due to the zeroth law of thermodynamics, it can be assumed that the room and all other objects in the room are in are also at the same temperature. Similar, to this example, if there is a glass of hot water, and a glass of ice water and they are placed on the countertop of the kitchen for few hours, then eventually they will reach a thermal equilibrium state with the room and everything will be at the same temperature.
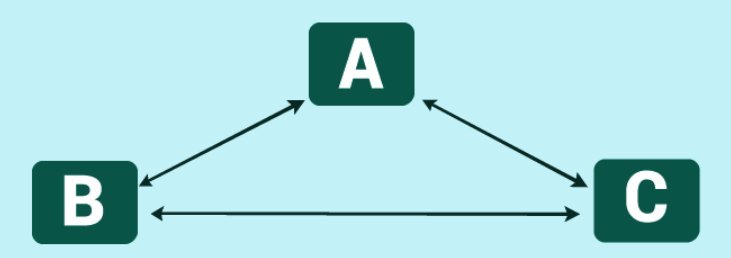
Zeroth Law as an Equivalence Relation
The set of all of the systems, in its state of the internal thermodynamic equilibrium, can be divided into different subsets in which each system will belong to the one subset only and it will be in thermal equilibrium with each member of that subset, but it will not be in thermal equilibrium with the members of any subsets. Due to this reason, a unique tag can be assigned to each system and if two systems have the same tag, then it shows that these systems are in thermal equilibrium with each other. If the tags are different, then it means that they are not in thermal equilibrium with each other. This property is being used for the justification of the use of empirical temperatures as the tagging system. Further, the relations of the thermally equilibrated systems are provided by the empirical temperatures such as continuity and order about the coldness and hotness.