Statement
For any dynamic system in equilibrium, according to the law of equilibrium, the full energy for the system is equally divided among the degree of freedom.
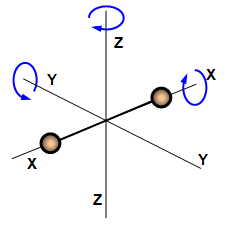
The kinetic energy of a single molecule along the x-axis, the y-axis and the z-axis is given as
, along the x-axis ![]()
, along the y-axis ![]()
, along the z-axis ![]()
Whenever the gas is at thermal equilibrium, the normal rate of kinetic energy is denoted as
,along the x-axis ![]()
, along the y-axis ![]()
, along the z-axis ![]()
In accordance to kinetic theory of gases, the normal kinetic energy of a molecule is given by,
![]()
, where Vrms is the root mean square velocity of the molecules, Kb is the Boltzmann constant and T is the temperature of the gas.The mono-atomic gas has three degrees of freedom, so the average kinetic energy per degree of freedom is given by
![]()
If a molecule is set free to move in space, it requires 3 coordinates to specify its accurate location, hence, it possesses 3 translational degrees of freedom. But if it is constrained to move in a plane, it possesses 2 translational degrees of freedom and if it is a straight line, it contains 1 translational degree of freedom. In case of a tri-atomic molecule, the degree of freedom is 6
Application of Specific Heat capacity
- Substances having a little heat energy capacity are often quickly heated, it also experiences a big change in temperature even though only a small amount of heat is supplied.
- Substances which contain a small specific heat capacity, are very useful as material in cooking things like frying pans, pots, kettles and so on, because, they can be quickly heated up even when a small amount of heat is provided to them.
- Sensitive thermometers must be made from materials containing small specific heat capacity so that it can truly detect and show the changes in temperature rapidly and accurately.
- Substances that usually contain the capability of high heat energy are more suitable as a material used for constructing kettle handlers, insulators and oven covers, because, a high amount of heat will cause only a little change in temperature and the material will not get hot too fast!
- Heat storage instruments are also very helpful and they are sometimes manufactured from substances that contain a high specific heat capacity.
- Water as a cooling agent acts wonderful as a cooling agent in engines. Water is additionally utilized in homes and in cold climate countries because as it is heated up (boiled). It tends to retain all the heat and warm up the house because of its high specific heat that capacity has.