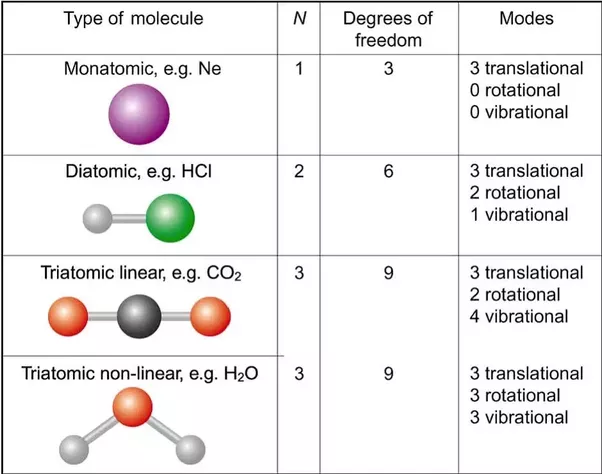
In three-dimensional area, 3 degrees of freedom are related to the movement of a particle. A diatomic gas molecule has half-dozen degrees of freedom. This set may be decomposed in terms of translations, rotations, and vibrations of the molecule; the center of mass motion of the entire molecule accounts for 3 degrees of freedom. Additionally, the molecule has two rotational degrees of motion with one vibrational mode. The rotations occur round the 2 axes perpendicular to the road between the 2 atoms; the rotation around the atom bond isn’t a physical rotation.
For a general, non-linear molecule, all 3 rotational degrees of freedom are considered, resulting in the decomposition.Which means that an N-atom molecule has 3N − 6 vibrational degrees of freedom for N > 2. In special cases, where are adsorbed large molecules, the rotational degrees of freedom can be limited to 1.
As outlined above one can even count degrees of freedom using the minimum number of coordinates needed to specify a position; this can be done as follows:
For a single particle, we need 2 coordinates in a 2-D plane to specify its position and 3 coordinates in 3-D space. Thus its degree of freedom in a 3-D space is 3.For a body consisting of 2 particles (ex. a diatomic molecule) in a 3D space with a constant distance between them. We can show its degrees of freedom to be five. Let’s say one particle in this body has coordinate (x1, y1, z1) and the other has coordinate (x2, y2, z2) with z2 unknown. Application of the formula for distance between two coordinates.
d=\sqrt
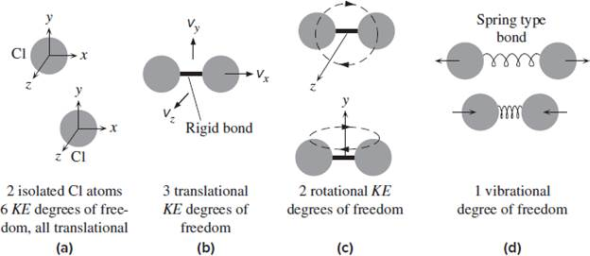
Absolute results in just one equation with one unknown, in which case we can solve it for z2. One of x1, x2, y1, y2, z1, or z2 can be unknown.Opposing to the classical equipartition theorem, the vibrational motion of molecules typically makes negligible contributions to the heat capacity at room temperature; that’s because these degrees of freedom are frozen as a result of the spacing between the energy eigenvalues exceeds the energy similar to ambient temperatures (kBT). In the following table such degrees of freedom are disregarded because of their low effect on total energy. Then solely the translational and rotational degrees of freedom contribute, in equal amounts, to the heat capacity ratio; this is why γ= 5/3
For monatomic gases and γ= 7/5
For diatomic gases at room temperature.
However, at very high temperatures, on the order of the vibrational temperature (Θvib), vibrational motion can’t be neglected.Vibrational temperatures mostly varies between 103 K and 104 K.