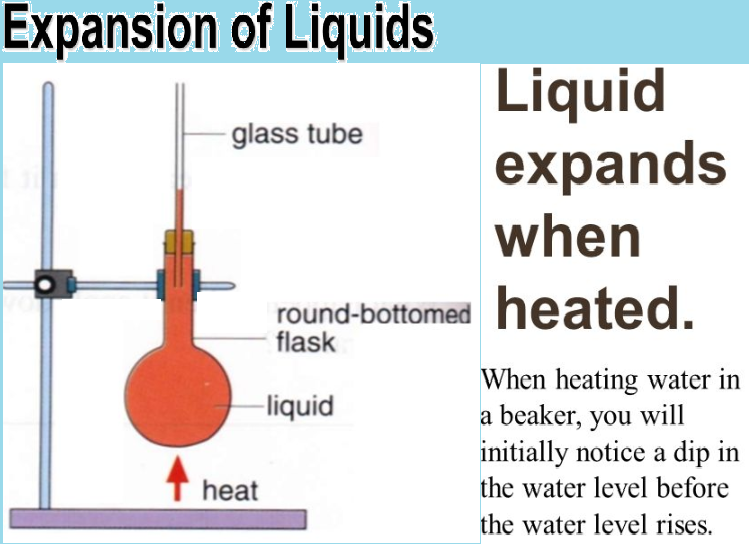
The liquids do not have any specific shape and they take the shape of the container in which they are placed. Thus, the liquids can be specified due to their volume. On heating of the liquid in the container, the heat is flowing to the liquid through the container which means that first there is an expansion in the container and the level of liquid falls due to this reason. When the liquid is heated, then it is heated beyond its original level. The intermediate state cannot be observed, but only the initial state and final state can be observed. Such an observed expansion of the liquids is an apparent expansion of the liquids. When the expansion of container is also considered and the total expansion in the volume of liquids is measured, then this expansion is called the absolute expansion of the liquids.
Real and Apparent Cubical Expansion of Liquids
Usually, the liquids are taken in the vessels, and upon eating both the liquid and the vessel are heated. Upon heating, the volume of the vessel is increased. By observation, it can be said that the increase in the volume of the liquid is less than the actual increase in the volume. The actual increase in the volume of liquid is known as the real cubical expansion of the liquids. The observed increase in the liquid’s volume excluding the vessel’s expansion is termed as the apparent cubical expansion of the liquids. This apparent cubical expansion of the liquids is always less than the real cubical expansion by the amount that is equal to the total cubical expansion of the liquids.
Coefficient of Real and Apparent Cubical Expansion of Liquids
The actual increase in the liquid’s volume of unit volume for the 1-degree rise in the temperature is known as the coefficient of real cubical expansion of that liquid. It is represented by the Yr. Whereas, the observed increase in the liquid’s volume, for a 1-degree increase in temperature, is known as the coefficient of the apparent cubical expansion, and it is represented by the symbol Ya.
Examples
The alcohol’s expansion in the thermometer is one of the commonly encountered examples of thermal expansion, as the volume of the given mass is increased with the change in the temperature. Due to an increase in the volume the hot air rises and due to this reason, the density of the hot air is smaller than the density of the air in its surroundings, thus causes the buoyant force on the hot air. The same process is followed by all the liquids.