d and f Block Elements
Alloy formation
Alloys are homogenous solid solutions of two or more metals obtained by melting and blending the components to produce a hardened solid obtained by cooling the melt. Alloys can be formed using metals with similar atomic radii. The atomic radii should not differ more than 15
The metals of first transition series show typical metallic character with ductility, malleability and hardness. These exhibit face-centred cubic (fcc), body-centred cubic (bcc) or hexagonal close-packed (hcp) type of lattice structures. These metals more than good conductors of heat as well as electricity. Copper and metals of the iron triad are softer than other metals. The common alloys of these metals are as follows: brass (Cu-Zn), nichrome (Ni-Cr),Monel metal (Cu-Ni), german silver (Cu-Ni-Zn), stainless steel (Fe-Cr-Ni-Mn), alnico steel (Fe-Ni-Co-Al), etc. These alloys are harder and have higher melting points than the parent metals. They are also more resistant to corrosion than their constituents.
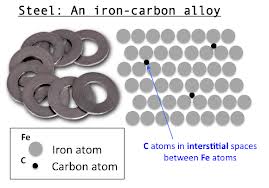
Fig : Steel: An iron-carbon alloy.
Iron forms mild steel with 1
Chromium steel (stainless steel + Fe + Cr +Ni) has good anti-corrosion properties and it is used for cutlery and chemical plant reactors. Nichrome is an alloy of chromium and nickel. It has a high melting point and a high electrical resistance and so it is used for electrical heating element wires.
The alloy Brass is produced by fuzzing copper and zinc together. It is a much more hard-wearing metal than copper (too soft) and zinc (too brittle) but is more malleable than bronze for ‘stamping’ or ‘cutting’ it into shape. The alloy Bronze is made from a mixture of copper and tin. It is stronger than copper and just as corrosion-resistant, eg used for sculptures. Copper is alloyed with nickel to give ‘cupro-nickel’, an attractive hard-wearing ‘silvery’ metal for coins. Aluminium is alloyed with copper, manganese and magnesium also it forms a very powerful alloy called duralumin. The alloy Solder is made from a mixture of tin and lead. It is a relatively low melting solid for electrical connections. The alloy Pewter mainly contains tin with small amounts of copper, bismuth (Bi) and antimony (Sb). It is stronger than tin but is easy to etch and engrave. Dental amalgam alloy is made by blending tin, mercury and silver with traces of other impurities. It has good anti-corrosion properties and resists the attack of acidic products produced by bacteria in the mouth.
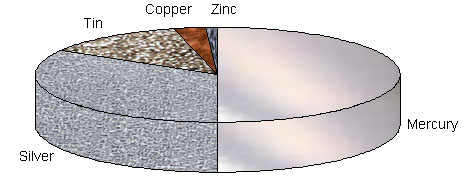
Fig : Conventional dental amalgam.