Water has a commonplace in our daily lives and it is regarded as the typical liquid. Water is one of the most unusual liquids that can be ever encountered. It is often said that life on the earth is dependent on the water, due to its properties. In terms of gasses, the water is one of the highest gas known, in liquids, it is denser even more than its solid form. At the same time, it is both slippery and sticky. The anomalous properties of the water cause huge bearings on human beings.
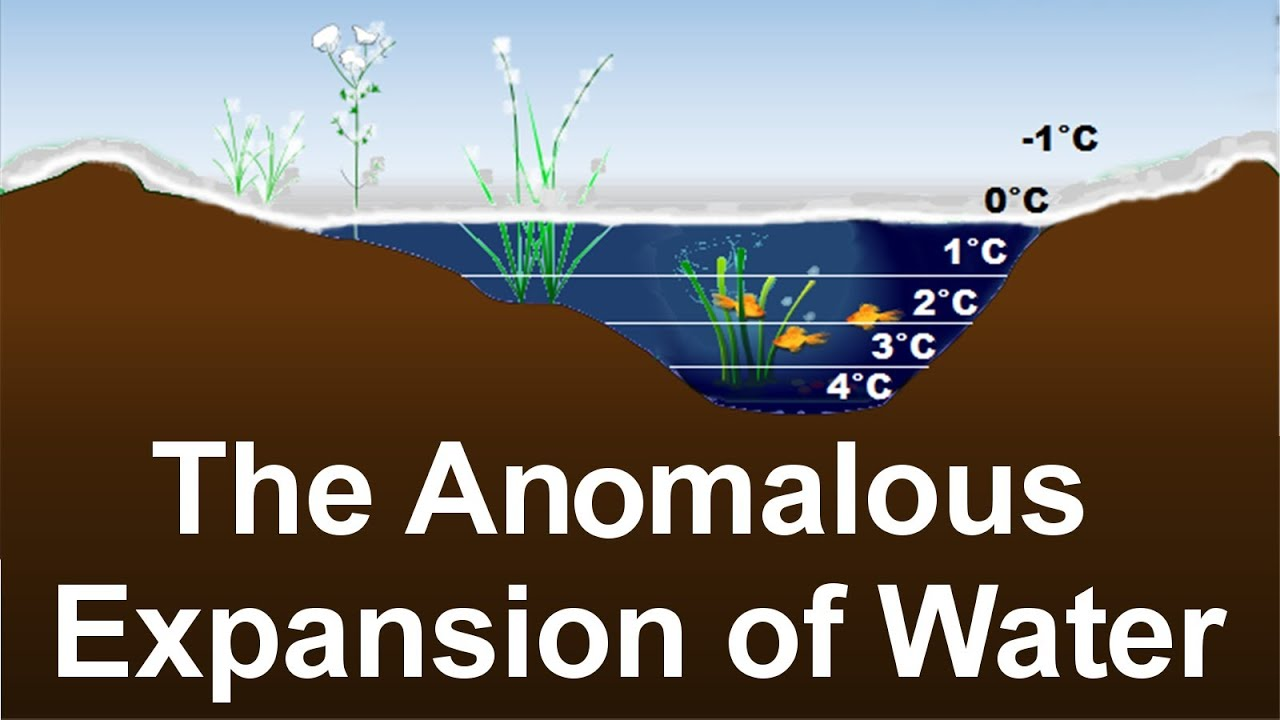
How Water Behaves Differently on Temperature Changes
Commonly the same trend is seen in the substances, that upon heating all the materials expand, and a decrease in the density is observed. The opposite phenomenon happens, when the materials are cooled, and an increase in the density is observed due to cooling. Upon cooling the water follows the general trend till the 4 degree Celsius, and gradually the density of the water is increased. The density is maximum when it reaches the 4 degrees Celsius. When further cooling is followed to make the ice such as at the 0 degree Celsius an expansion in the water is observed with a further drop in the temperature.
Why Water Behaves Differently
The water molecule is made up of two hydrogens and one oxygen atom. At the normal temperature, the molecules of water are held together in the liquid state due to the intermolecular attraction between the water molecules. In the liquid state, the water molecules are constantly rearranged and are continuously whizzing around in the container. Upon cooling the rate of whizzing around is decreased as they lose their energy. On, further cooling, the squeezing of water molecules is observed and density is increased. The density is maximum at 4 degrees Celsius and further, there is no squeezing. Now the freezing water is held by hydrogen and oxygen attraction. Due to the lattice arrangement water movement is prevented. But the bonding between the hydrogen and oxygen is not tight as between the O-O, so little expansion is observed and water behaves anomalously due to these characters.
Freezing of Top Layer Protects the Aquatic Life
Due to the expansion of the water, the coolest water is always on the surface. Since the water, at the 4 degrees, Celsius is the heavier so this water is settled at the bottom of the water body, and the coolest layer is accumulated on the top layer. Due to this reason, the freezing is always observed from the top layer, while the aquatic life can live under the liquid layer. As both ice and water are bad conductors of heat, so the top layer causes the insulation of the rest of the water body, from the cold winter and in this way, the aquatic life is protected.