Boron is a chemical element. It takes the symbol B in the periodic table and its atomic number is 5. Boron is also found in the solar system and earth’s crust as a product of cosmic ray spallation. It is a dark brown substance with an atomic mass of 10.81. Boron appears in period 2 of the periodic table and its electron configuration is
[He] 2s^2 2p^1
It is found in group 13, the boron group and contains 2, 3 electrons per shell. Here is how it looks in mineral form.

Physical properties of boron
- It is a solid at STP
- It has a melting point of 2348k (2076^0c, 3769^F) and a boiling point of 4200k (3927^c, 7107^F).
- When in molten form or in liquid state at melting point, Boron has a density of 2.08g/cm^3.
- Total amount of heat of fusion needed to change it from solid form to liquid form is 50.2 kJ/mol and the heat of vaporization needed to change it from liquid to gas is 508kJ/mol.
Atomic properties
- The atomic radius of Boron is is an empirical number of 90 pm while its covalent radius is 84+-3.
- It uses the weak van der Waals forces to make a bond between its atoms.
- It is a mildly acidic oxide which can be found in the following oxidation states; -5, -1, +1, +3
- Boron has three levels of ionization energies namely
1st: 800Kj/mol
2nd:2427kJ/mol
3rd:3659kJ/mol
Other properties
- Boron is a crystalline element and found naturally in its primordial form and takes the shape of a rhombohedra.
- It travels in a speed of sound of 16200m/s and at a constant state at 20^0c.
- It is a highly conductive substance. The thermal conductivity of boron is 27.4W (m.K) and it contains a high degree of electrical resistivity.
The historical properties
- It was first discovered by Joseph Luis Gay-Lussac and Louis Jacques Thenard on 30th June I the year 1808
- Boron then went through the process of isolation from other elements in July 9th 1808
- The word boron first came from the word borax. Borax was the mineral from which the element was first isolated from. Boron is a compound consisting of carbon elements.
- When boron is oxidized by air, the two-scientist proved that boric acid is a by-product of oxygen and Boron chemical compound.
Characteristics
- It is very similar to carbon in its ability to form molecular networks of stable covalent bonds.
- Amorphous Boron has many regular boron atomic that bonded together by weak van der Waals forces.
- The crystalline form of Boron is a hard black material that has a boiling point above 2000^o c and cannot produce pure polymorphs that are uncontaminated.
Facts about Boron
It is rare to find elemental boron and the element is hard to study because no pure form of the element is easy to prepare.
The chemical behavior of the element has a lot of similarities to that of silicon and aluminum. Boron in its solid state does not react with hydrochloric acid but when it is reacted with concentrated hydrogen peroxide, nitric acid or sulfuric acid, it forms different forms of acids.
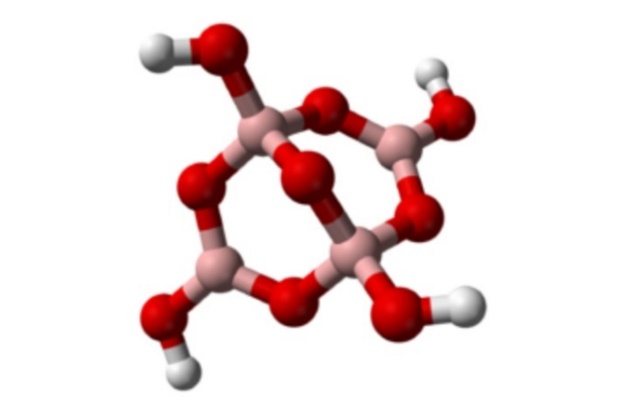
Boron do not react with oxygen at room temp but at a higher temperature it reacts to form boron trioxide. Below is a figure showing boron trioxide.
Applications
- Used in the production of glass and ceramics
- Used in manufacture of golf clubs and fishing rods
- Boron manufactures boron carbide that is used in explosives
- Used in production of abrasives
- Boron is used in hardening steels and alloys