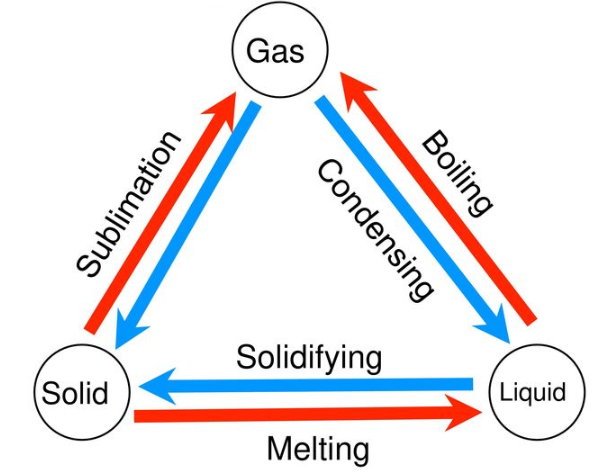
The matter is always in the changing states and the changes in the state of the matter are the physical changes. These changes are reversible and there is no involvement of the chemical properties or the chemical makeup. The common changes of the state include the freezing, melting, deposition, sublimation, condensation, and vaporization.
Temperature, Energy, and Changes of State
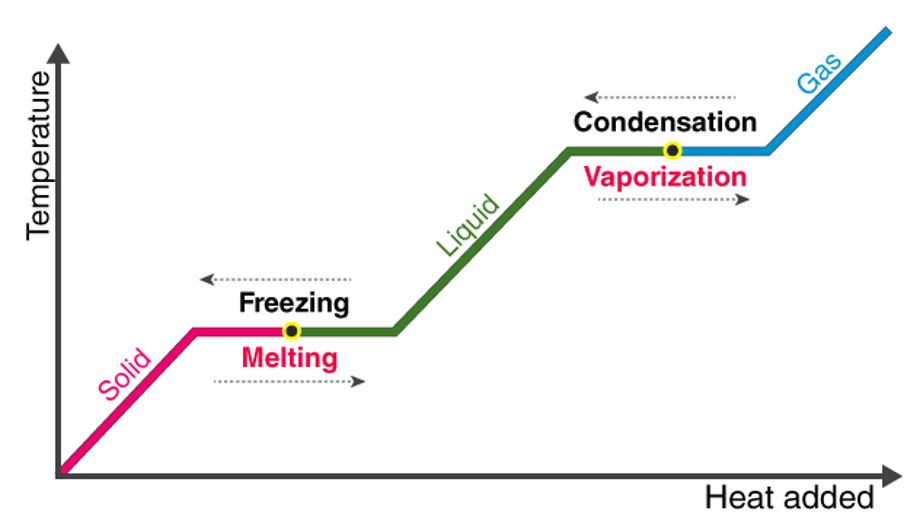
Energy is always associated with the changes in the state. The matter either absorbs energy or losses the energy when it follows the changes in its state. When a solid is changed into the liquid, then the matter must have to absorb the energy from its surroundings. By using the thermometer, the amount of energy in the matter can be measured. The reason behind this is that the thermometer is used for the measurement of the temperature and the temperature is the average kinetic energy that is associated with the particles of the matter.
Changes Between the Liquids and Solids
The changes between the liquid and solid-state of the matter can be observed due to the freezing and melting. In the freezer, the warmer water loses the heat to the colder air. The water cools until the particles do not have enough energy to slide past each other. Instead, they remain locked in the place in the fixed positions due to the forces of attraction between them. In this way, the liquid water is changed to solid ice. When the ice cubes are taking out of the freezer and they are left in the warmer room, then the energy is absorbed by the ice from the warmer air around its surroundings. By this energy, the particles of the frozen water can overcome the forces of attraction between them. Resultantly, they will be able to slip out from their fixed positions and the ice will be converted to the water.
Changes Between the Liquids and Gasses
The changes in the state between the liquids and the gases happen due to vaporization, condensation, and evaporation. On getting enough heat the water starts getting boiling. In the boiling water, the bubbles of the water vapors are formed. When the particles in the liquid water gets enough energy to overcome the forces of attraction, they are changed to the gaseous state. When the steam cannot escape out of the room, then it is cooled back and, in this way, the gas is again changed to the liquid.
Changes Between the Gasses and Solids
Solids can change to the gasses without passing through the liquid state by the process of sublimation. The opposite of the sublimation also happens by the deposition when the gas is directly changed to the solid.