All matter in this universe is made up of elements and each element is considered as a chemical. Elements interact with each other and form bond to make molecules. When these molecules interact with each other then reactants are changed into products and this phenomenon is termed as chemical reaction. These are the reactions by which chemicals interact with each other and new chemicals of different compositions are made. Chemical reaction is also termed as chemical change. In simple statement, this process transforms the reactants to the products. However, the rate of chemical reaction and transformations is strongly dependent on chemical properties of compounds and elements.
Where Chemical Reactions Occur
Since the beginning of earth and life, chemical reactions are occurring everywhere and scientists understood them in the 18th century. Fermentation process that basically convert the sugar to alcohol is well known phenomenon for centuries but the basis of this involved chemical reaction were not known. Chemical reactions are occurring everywhere, even they are involved in cleaning, cooking, and body growth. Some common examples of chemical reactions are photosynthesis, aerobic cellular respiration, aerobic respiration, combustion, rusting, metathesis, digestion, electrochemistry, acid base reactions, detergent and soap reactions, and changes in food while cooking etc. Metabolic pathways in human cells, and rusting of iron all are examples of the chemical reactions.
Sometimes, people may associate the chemical reactions with sterile environment of test tube and laboratory but it is not so. In our surroundings chemical reactions are occurring constantly. Chemical reactions are less controlled in nature as compare to the laboratory conditions.
What Happens During Chemical Reactions
Typically, a chemical reaction is represented by chemical equation which exactly represents the change of reactants to the products. It shows the products on right hand side and reactants on left hand size. Stoichiometric coefficients are used for typical chemical reaction to show relative amount of the reactants and products involved in the chemical reactions.
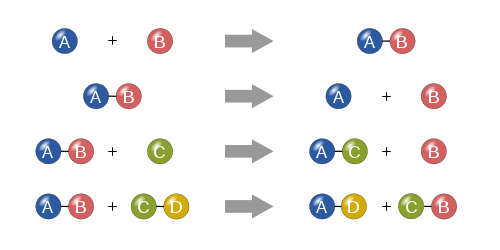
During chemical reactions, physical changes occurs but the nature of substance is not changed. The common physical reactions that occurs during chemical reaction are evolution of gas, scent of gas, and change in color of the substance. Chemical reactions are much significant to give clear understanding of properties of matter.
Chemical reactions lead to the formation of new materials which are totally different from the reactants. Just mixing of substances cannot be stated as chemical reaction. Not always but often heat is essential for start of chemical reactions and most of our energy is produced by the chemical reactions. Chemical reactions are widely used to identify, analyze, and test different materials. For all chemical reactions to occur, oxygen is much important and causes rusting and combustion. Chemical reactions are also involved in the break-down of food that we eat and produces energy in the form of ATP for functioning of body.
Types of Chemical Reactions
There are many types of chemical reactions and these includes following.
- Double replacement or precipitation reaction
- Neutralization or acid base reaction
- Redox or oxidation reduction reaction
- Synthesis reactions
- Combustion reactions
- Single replacement reactions
- Decomposition reactions