Substitution Reactions of Diazonium Ions
In the solutions of benzene diazonium chloride, diazonium ions are present. They have one N2+ group and in benzene diazonium chloride, it is attached to the benzene ring. In a set of reactions of diazonium ions, this group is replaced by something else, causing the release of nitrogen gas.
Substitution by a Hydroxyl Group
For this reaction, a warm solution of benzene diazonium chloride is needed. In the solution, diazonium ion reacts with the water and phenol is produced that is either a black oily liquid or in the solution form but its quantity is dependent on the conditions. In this reaction, nitrogen gas is evolved. This reaction is the same as the reaction of phenylamine with the nitrous acid in the warmer conditions. At first, diazonium ion is produced and, in the solution, it immediately reacts with the water to make the phenol.
Substitution by an Iodine Atom
This reaction is a good example of the use of diazonium salts for substituting to the benzene ring which is otherwise much difficult to attach. The nitrogen gas is given off by the addition of potassium iodide solution to the solution of benzene diazonium chloride in the cold conditions. Oily drops of iso benzene are formed in this reaction. The reaction between the iodide ions and the diazonium ions is simple and resultantly potassium iodide solution is formed.
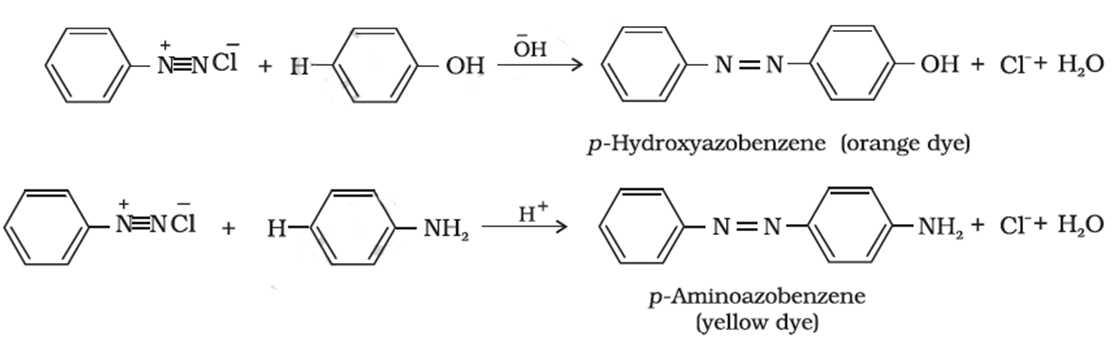
Coupling Reactions of Diazonium Ions
Nitrogen diazonium ion is lost in the substitution reactions but in this reaction, nitrogen is retained and makes the bridge between the two benzene rings.
Reaction with Phenol
In the solution of sodium hydroxide, phenol is dissolved and as a result, a solution of sodium phenoxide is produced. A cold solution of benzene diazonium chloride is added and a yellow-orange precipitate or the solution is obtained. The product is the simplest azo compound where nitrogen bridges the two benzene rings.
Reaction with Phenyl Amine
To a cold solution of benzene diazonium chloride, some liquid phenylamine is added the mixture is vigorously shaken. Resultantly, a solid of yellow color is produced. Frequently these compounds are used as azo dyes.
Reaction with naphthalene-2-ol
The naphthalene compound has two benzene rings which are fused with each other. The reaction conditions are the same as the reaction conditions of the phenol. In the solution of sodium hydroxide, naphthalene-2-ol is dissolved and an ion like the phenol is produced. The resulting solution is cooled and then it is mixed with the benzene diazonium chloride solution and an orange-red precipitate of intense color is produced.