d and f Block Elements
Comparison with lanthanides
Similarities
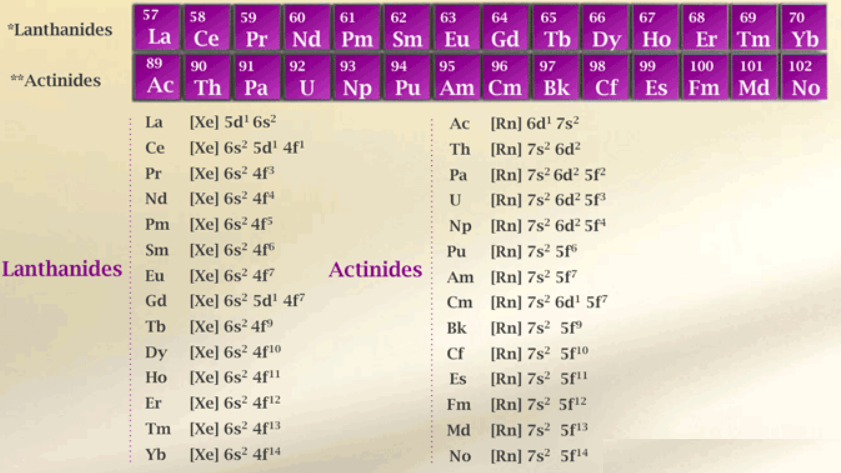
In the atoms of the elements of both the series, three outermost shells are partially filled and remaining inner shells are completely filled but the additional or differentiating electron enters (n-2) f-subshell. The elements of both series exhibit +3 oxidation state which is a prominent and predominant state. Like Lanthanide contraction found in the lanthanide elements, there occurs contraction in size in the actinide elements called actinide contraction. Both the contractions are due to poor shielding effect produced by f-electron with increasing nuclear charge. The elements of both the series are quite reactive and are electropositive. The electronic absorption bands of the elements of both the series are sharp and appear like lines. These bands are produced due to f-f transitions within (n-2)f subshell though such transitions are orbital forbidden. Most of the lanthanide and actinide cations are paramagnetic. The nitrates, perchlorates and sulphates of trivalent lanthanide and actinide elements are soluble while the hydroxides, fluorides and carbonates of these elements are insoluble. The lanthanide and actinide elements show similarity in properties among their series though the lanthanides are closer among them sieves in properties as compared to actinides.
Differences
| Properties | Lanthanides | Actinides |
| Electronic configuration | [Xe] 4f1‐14, 5d0‐1 ,6s2 | [Rn] 5f1‐14, 6d0‐1 ,7s |
| Electron occupancy | In lanthanide, the newly added electron enters in 4f- orbitals. | In actinide, the newly added electrons are occupied in the 5f- orbitals |
| Atomic/ionic sizes | Size decreases from La to Lu, and the size is more than actinides. | Size decreases from Ac to Lw. The atomic and ionic size is smaller than lanthanoids due to more shielding effect in the 5f orbital. |
| Oxidation states | Common oxidation is +3 where other oxidation states are +2, +4. This is possible because of a large energy gap between 4f, 5d and 6s subshell | Common oxidation is +3 where other oxidation states are +2, +4,+5 and+7 due to due to the small energy difference between 5f, 6d and 7s orbitals |
| Chemical reactivity | The earlier member quite
reactive but with increasing the atomic number they behave like aluminium. |
The actinides highly reactive, especially in finely divided. |
| Complex
formation |
Less tendency to form complex ions due to less charge density. They form a complex with a ligand having oxygen or oxygen plus nitrogen like glycine, oxalate etc. | More tendency to form complex ions with π-accepter ligand and anions due to high charge density. |
| Binding energy | They have less binding energy, hence less shielding effect in 4f- orbital. | They have more binding energy which is the result of more shielding effect in 5f- orbital. |
| Colour | Their colour absorptive spectra are less intense than actinides | Their colour absorptive spectra are more intense than lanthanides. |
| Magnetic properties | They have more magnetic moment than the
actinides |
They have less magnetic moment than the
lanthanides. |