p-Block Elements: Group 16
Compounds of Sulphur
| Chemical formula | Name | Oxidation state |
| S2-, H2S | Sulphide | -2 |
| S8 | Sulphur | 0 |
| SCl2, S2O32- | Sulphur chloride and oxide | +2 |
| SO2, SO32-, H2SO3 | Sulphite | +4 |
| SO3, SO42-, H2SO4 | Sulphate | +6 |
Sulphides, S2-:
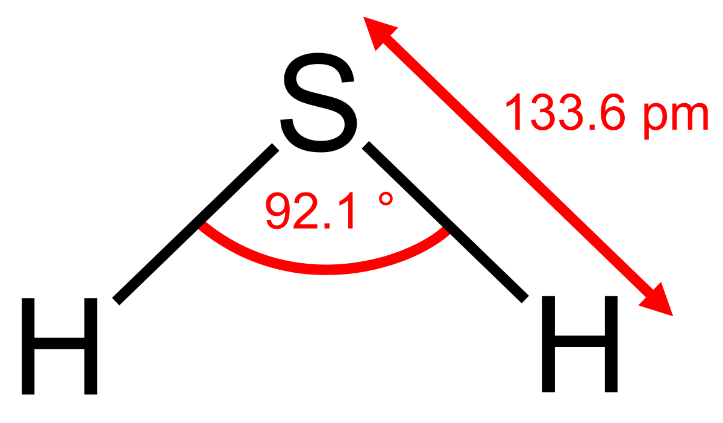
Hydrogen sulphide, H2S:
It is a colourless acidic gas found in vapours from a volcano and formed when organic matters decay. It is prepared in the laboratory by treating sulphide with an acid.
FeS(s) + 2HCl (aq) FeCl2(aq) + H2S (g)
Carbon disulphide, CS2: When sulphurvapour is passed over highly heated carbon the two elements combine, forming carbon disulphide (CS2), just as oxygen and carbon unite to form carbon dioxide (CO2). It is a heavy, colourless liquid, possessing and pure gas has a pleasant ethereal odour. On standing for some time, especially when exposed to sunlight, it undergoes a slight decomposition and acquires a most disagreeable, rancid odour. It has the property of dissolving many substances, such as gums, resins, and waxes, which are insoluble in most liquids, and it is extensively used as a solvent for such substances. It is also used as an insecticide. It boils at a low temperature (46° C), and its vapour is very inflammable, burning in the air to form carbon dioxide and sulphur dioxide, according to the equation
CS2 + 3O2 CO2 + 2SO2.
Most metallic sulphides are abundant in nature and are found as ores. These sulphides are insoluble in water. For example:
Selenium disulphide, Se2S:
Hydrogen sulphide is passed through selenious acid to produce this compound. It is a bright orange powder with a characteristic faint sulphideodour. It is insoluble in water, alcohol and organic solvents. This compound is mainly used in anti-dandruff shampoos and antifungals.
Sulphites, SO32-:
The sulphites have the power of taking up oxygen very readily, and are good reducing agents. When sulphur dioxide dissolves in water it combines chemically with it to form sulphurous acid, an unstable substance having the formula H2SO3. Preparation of this acid in pure form is very difficult because it breaks down very easily into water and sulphur dioxide. This acid has acidic and reducing properties. The reaction for the production if acid is reversible, and is expressed by the equation
H2O + SO2↔ H2SO3
Sulphates, SO42-:
The sulphates make up a very important class of salts. Many of these salts have commercial uses while others make mineral ores. Important minerals include Copperas (iron sulphate), blue vitriol (copper sulphate), and Epsom salt (magnesium sulphate) are used commercially while others include gypsum (calcium sulphate) and barytes (barium sulphate) which are used in medicinal purposes. Sulphates can be tested by adding a solution of BaCl2 acidified with dilute hydrochloric acid and produce a white precipitate.
Ba2+ + SO42-BaSO4
Thiosulphuric acid, H2S2O3: Thiosulphuric acid can be prepared by using sodium sulphiteand boiled with Sulphur. The two substances combine and form a salt:
Na2SO3 + S Na2S2O3.
This compound is called sodium thiosulphate. It is a salt of the easily decomposed acid H2S2O3, called thiosulphuric acid.