p-Block Elements: Coordination compounds Colour
Transition metal ions show many intense colours in host crystals or solution. The colour of light absorbed by the complexed ion is related to electronic energy changes in the structure of the complex.

Crystal field theory
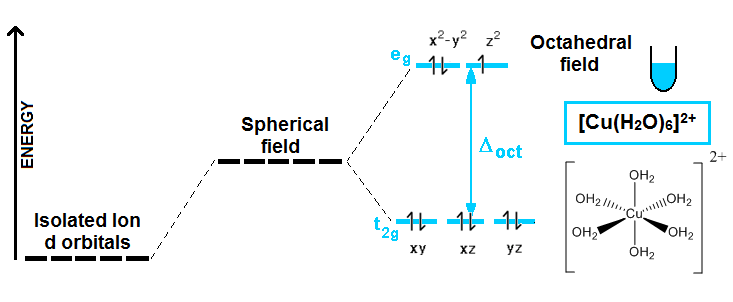
Crystal field theory assumes that the interaction between the metal ion and the ligand is electrostatic. The ligands are able to split the energies of the transition metal d orbitals into lower and higher energy states (Fig. 3). Visible light will excite an electron from a lower energy d orbital to a higher energy d orbital. This accounts for many of the properties of coordination compounds, such as colour and magnetic properties. The colour of coordination compounds depends on both the metal and the ligand. In a spectrochemical series, ligands are arranged in order of their increasing energy gap between the lower and higher energy d orbitals.
In crystal field theory one assumes that the ligands can be represented by negative point charges and that the metal is a positive point charge located at the centre of the system. One then examines how these negative point charges interact with the d orbitals. When all five electrons are unpaired, they have week field while one unpaired electron in the ligand shows strong field. For example, [Mn(CN)6]4- is more likely to be high spin because the charge on the Mn ion is 2+ while the charge on the Mn ion is 3+ in the other complex. With a larger charge, the splitting between energy levels is larger hence creating a strong field or low spin.
Fig : Crystal field theory
The energy gap between the eg and t2g orbitals, ∆0, (the crystal field splitting) equals the energy of a photon:
∆0= hν = ∆E.
As ∆0, varies, hν will also vary and the colour of the Absorption of a photon causes a compound will change. The observed colour is the complementary colour of the one that is absorbed. The colours of complex ions are due to electronic transitions between the split d sublevel orbitals. The wavelength of maximum absorbance determines the size of the energy bandgap between the split d sublevel orbitals. When no light is absorbed, all reflected to get the white colour and when all light is absorbed, none reflected to get the Black colour
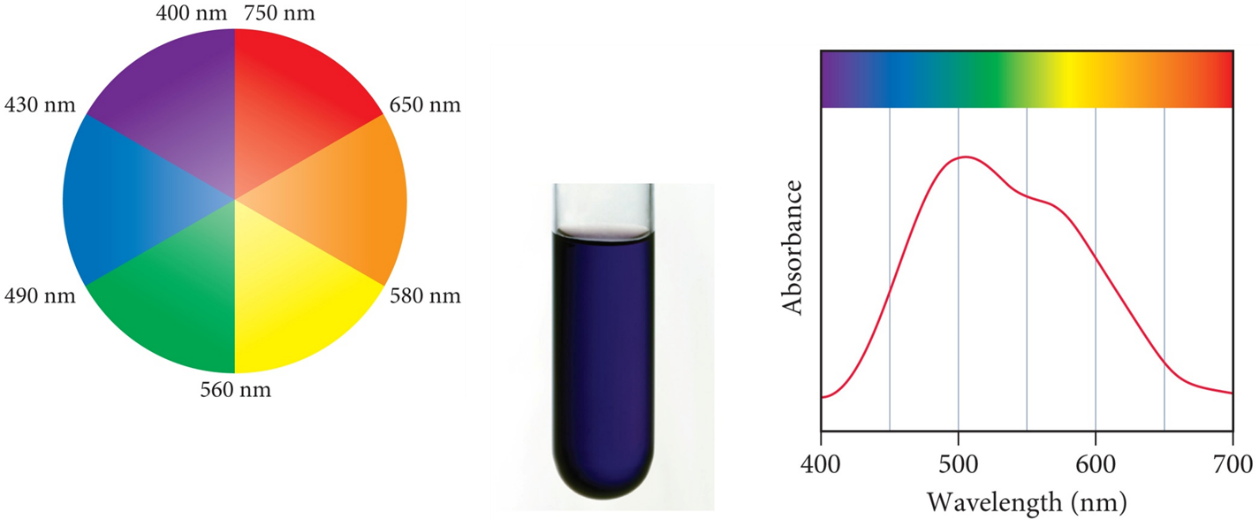
Fig :Spectroscopic measurements of ∆ allowing ordering of ligands ability to split the d orbitals called a spectrochemical series.
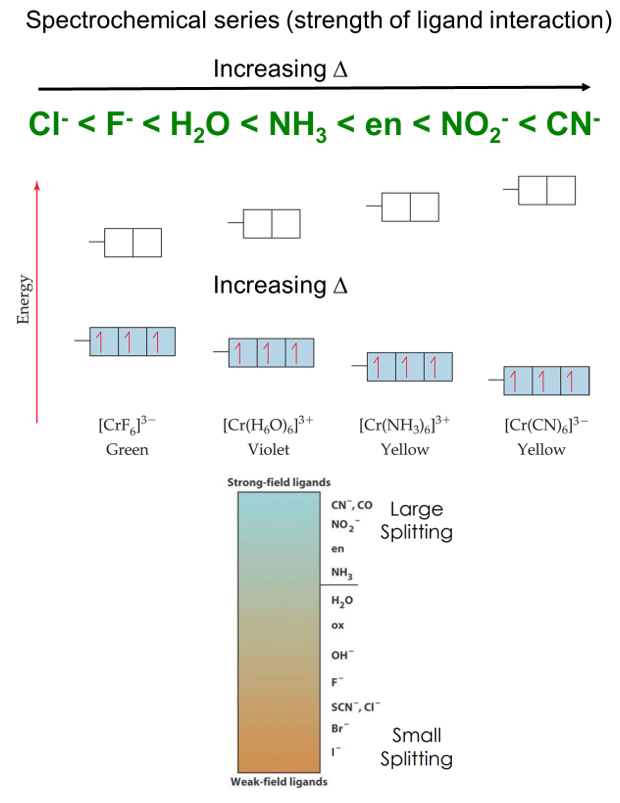
Different ligands affect the d orbitals of a given metal ion to different degrees and thus produce different values of the ligand field splitting. The spectrochemical series helps in placing the ligands according to the relative magnitudes of the ligand field splitting.
The size of the energy gap depends on what kind of ligands are attached.
-
- Strong field ligands include CN─> NO2─>en> NH3.
- Weak field ligands include H2O > OH─> F─> Cl─> Br─> I─.
The size of the energy gap also depends on the type of cation or metal in the complex.
-
- Increases as the charge on the metal cation increases
- Co3+> Cr3+> Fe3+> Fe2+> Co2+> Ni2+> Mn2+
- ∆o increases with increasing oxidation number on the metal.
- Mn+2<Ni+2<Co+2<Fe+2<V+2<Fe+3<Co+3<Mn+4<Mo+3<Rh+3<Ru+3<Pd+4<Ir+3<Pt+4
- ∆o increases with increases going down a group of metals.
Crystal Field Theory does not consider the nature of the ligand. Likewise, it cannot explain the spectrochemical series. Alternatively, the Ligand Field Theory uses a molecular orbital approach. It considers that in the initial stage, the ligands have a hybrid orbital or a p orbital pointing toward the metal to make σ bonds. THE hybridized orbital then has a different energy level which differentiates the energy gap between the transition state from one state to another.