p-Block Elements: Coordination compounds
Coordination number
The coordination number for a metal ion in a complex is the number of ligands that are directly attached to it. Mostly coordination numbers of 4 and 6 are observed, and 5 is of considerable interest, though coordination numbers can range from 2 to 12 and beyond.
Coordination number 2
The stoichiometry of these compounds is ML2. Examples: Cu(NH3)2+, AgCl2– , Au(CN)2 – , HgCl2
These are linear and the ligand-metal-ligand bond angel is 180° with minimum ligand-ligand repulsion; Cl – Ag – Cl. These metal ions have d10 configuration in the ground state. Even though the metal-ligand bond may be considered to be formed due to the overlap of a σ-orbital of the ligands with sp-hybridized metal orbital, actually some d-orbital contribution is also there in the bond formation.
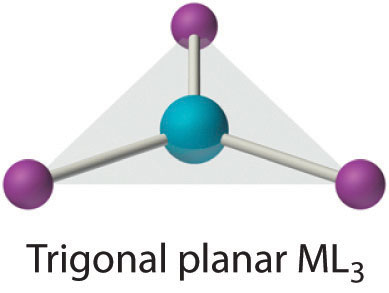
Coordination number 3
It is a rare coordination number. In many crystalline compounds, the stoichiometry may be ML3but the actual coordination number may be greater than 3. When the ligands are extremely bulky, this coordination number exists. In some of the d10 systems, this coordination number exists, even when the ligands are not bulky. Examples: KCu(CN)2, Pt(PPh3)3 etc. In such complexes, the metal atom and the ligands directly connected to it lie on the same plane.
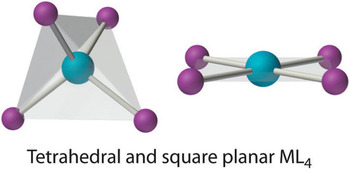
Coordination number 4
This is one of the most common and important coordination numbers. A metal ion can connect with four lone pair in two shapes:
Tetrahedral:
If the central metal atom is not having any lone pair of electrons and if it is not a transition metal, then it will form a tetrahedral complex. BeCl42- , BF4– , SnCl4, AlF4– etc.
On the other hand, if the central metal atom is a transition metal but it does not have a d8 configuration, then it forms a tetrahedral complex. e.g. the tetrachlorocopper(II) ion, [CuCl4] 2-,FeCl4– , CoCl42- , MnO4 – etc.
When the central metal atom hasd8 configuration, usually it will form a square planar complex but sometimes it can also choose to obtain a tetrahedral complex structure. Example: NiCl42- , NiCl3OPPh3. When the ligands are bulky, Co(I) and Ni(II) form tetrahedral complexes.
Square planar:
Square planar,If the central metal ion has a d8 configuration, mostly it will form square planar complexes e.g. the tetrachlorogold(III) ion, [AuCl4] – Ni(CN)42-, PdCl42-, PtCl4 2- etc., where the internal bond angles are all 90°.
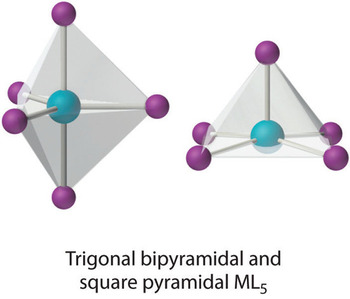
Coordination number 5
Two shapes are
- Trigonal bipyramid, e.g. pentachloro-cuprate(II), [CuCl5] 3-
- The square pyramid, e.g. pentacyano-nickelate(II), [Ni(CN)5] 3-
The distortion of a trigonal bipyramidal structure creates a square pyramidal structure. Examples: CdCl53- has a trigonal bipyramidal structure. Ni(CN)53- has got a square pyramidal structure.
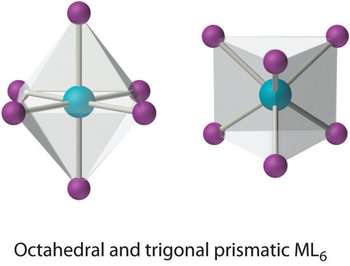
Coordination number 6
Complexes of the type, MA6 has octahedral coordination and the symmetry is Oh have a coordination number 6. Other complexes of the type, MA5B, MA4B2, etc. are also known as octahedral complexes. There is only one shape available for a metal that is enclosed by six lone pairs – octahedral, e.g. the Hexa-aquocopper(II) ion, [Cu (H2O)6] 2+