Generally, D and L notation is used to describe the stereochemistry of carbohydrates. This system is named after the Latin dexter and laevous that stands for the right and left. It is used to name the molecule by relating them to the glyceraldehyde molecule. To differentiate between the D and L carbohydrates a Fischer’s projection is used. For the Fischer’s projection of monosaccharides, the penultimate carbon that is next to the last one of D sugars is depicted with the hydrogen on left and hydroxyl on right. L sugars are shown on hydrogen with the right and hydroxyl on the left side. Cyclohexane structure of hexoses, in the standard Haworth projection, have the typical pointing up terminal carbon. Fischer’s projection of the L and D carbohydrates illustrates the relationship between the L and D on the penultimate carbon.
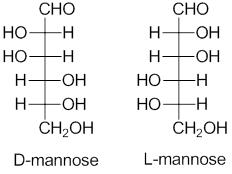
D configuration is given to the carbohydrates if the hydroxyl group is placed to the right of the last stereocenter in the Fischer’s projection and the L configuration is given if the hydroxyl is placed on the left of the last stereocenter carbon. Usually, L or D is put at the beginning of the carbohydrates while giving names to the molecules. It only gives the configuration of the carbohydrates and does not specify anything relating to the sign of the rotation of the plane-polarized light and the molecules in which atoms have different structural arrangements are asymmetrical molecules.
Absolute stereochemistry is not designated by the D and L configuration but it can be determined by the anomeric carbon center by comparing its orientation to the glyceraldehyde. D-L system is important as it gives the relative configuration of the molecules. This system corresponds to the molecular configuration by the spatial arrangement of the atoms of the molecule around the chirality center. Sometimes + and – notation is also associated with the D-L configuration system which shows the rotation of the plane of polarized light that either it is clockwise or anticlockwise. However, this system does not have any connection to the + or – notation. This system is based on the comparison of the configuration of the molecules to the enantiomers of glyceraldehyde. Specifically, it is used in the realm of amino acids and sugars. Though this is an old system, dating back to the horse and buggy era, when Emil Fischer worked on carbohydrates in the 1800s, still it is used in the modern era.