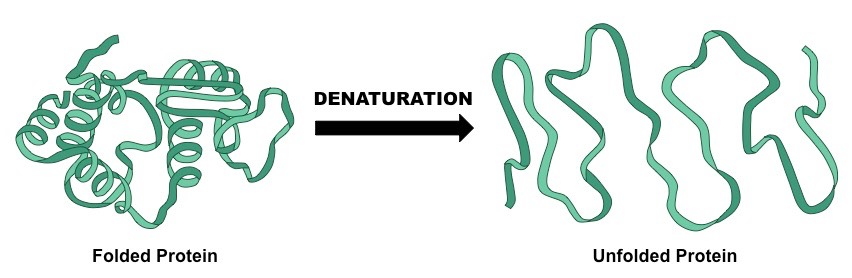
The term denaturation of proteins is used for the possible disturbance and disruption of its secondary and tertiary structures. The denaturation reactions are not much strong, and a peptide bond is not broken, so the primary structure remains the same even after the denaturation. Due to the denaturation, the normal alpha-helix, ad beta-sheets are disrupted and the protein’s structure is uncoiled into the random shape. Denaturation occurs due to the bonding interactions, which are responsible for the disruption of secondary structure, and tertiary structure. The structures of the proteins are highly organized and are a true masterpiece of the chemical architecture but they have a certain delicacy and depending on the conditions, they are denatured. By the term denaturation, it means that there is a change in the three-dimensional structure of the proteins due to which they become incapable to perform their targeted functions.
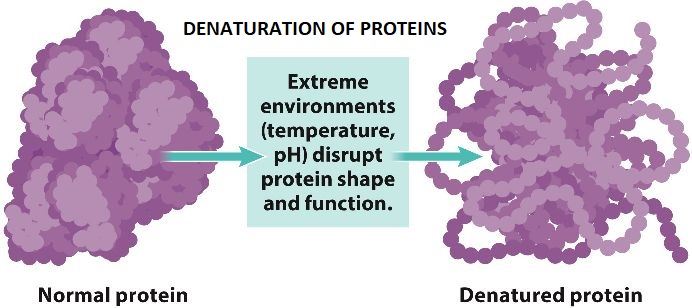
Sometimes, the phenomenon of denaturation is equated with the coagulation or precipitation of the protein. A wide range of conditions, such as pH changes, heat, reagents, organic compounds, and the ions of heavy metals can cause the denaturation of the proteins. On boiling the protein solution, the protein is denatured and frequently, it becomes insoluble. Even after the solution is cooled, it remains insoluble. When an egg is boiled or heat, then the white part of the egg is denatured and this is a good example of the irreversible denaturation. While cooking the food, some of the proteins are denatured. When thermally unstable whites are cooked, they are turned as opaque and interconnected solid mass is formed. Another example of the denatured protein is the skin that makes the curdled milk.
The structure of a denatured protein is the same as that of the original one. At the elevated temperatures, weak forces present between the charged groups are disrupted and resultantly, the tertiary structure of the proteins is lost. In some circumstances, it is possible to regenerate the original structure of the proteins. A wide range of the characteristics is exhibited by the denatured proteins such as communal aggregation, and the loss of solubility. Upon denaturation, the biological functions of most of the proteins are lost. The activity of the enzymes is lost as their substrates will no longer be able to bind to their relevant active site and it happens due to the reason that the amino acid is no longer positioned to perform the activities normally. This denaturation can be induced due to biological factors or due to the chemical agents.