d and f Block Elements
Lanthanides: Electronic configuration
The third transition series (inner) or 4f series contains 15 elements from Lanthanum (La) to Hafnium (Hf) through Gold (Au)) with 5d subshell being filled with electrons.
The lanthanides are soft metals similar to the first period and they can be easily cut with a knife. The elements are hard to separate when they occur in the same ore because they have similar atomic radii. The elements are given the name rare earth, but the lanthanides are not as rare as originally thought. Cerium makes up 50% of an alloy called Misch (MIHSH) metal. Flints in lighters are made from Misch metal.
The general electronic configuration of these elements is [Xe] 4f0-14 5d 0-1 6s2 where 4f orbital is gradually filled as the atomic number increases.
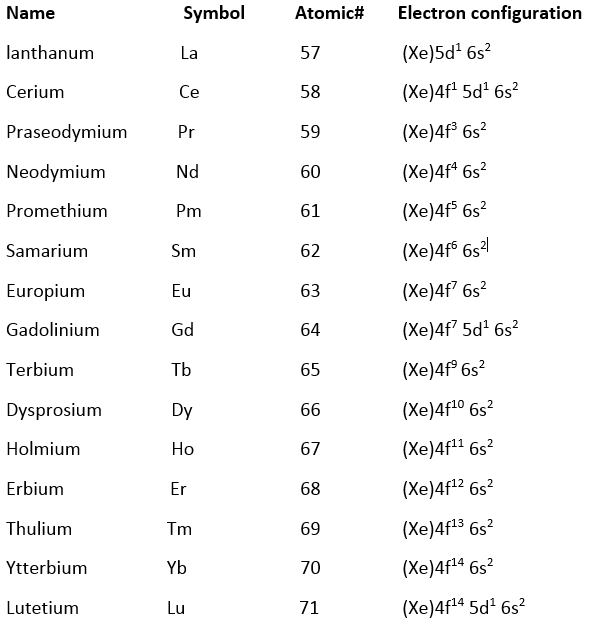
From the above electronic configuration, the Lanthanum, La has 5d orbital singly occupied but after La further filling of 5d orbital is discontinued. As the nuclear charge increases by one unit from La to Ce, 4f orbitals were higher in energy upto Lu, fall slightly below the 5d level 4f- orbitals, therefore begin to fill and are completely filled up to Lu, before filling of 5d orbital is resumed.
Points may be noted from above configuration:
The valence shell configuration is 4f 0,2-14 5d0 or 1 6s2. This configuration indicates that the additional electron enters the 4f level without altering the electrons in the 6s- orbital. The filling of 4f- orbitals are not regular. This means that the additional electron in Gd does not enter 4f- orbital but it goes to 5d level. This is because the 4f & 5d orbital in Gd are at about the same energy level and Gd atom tends to retain the configuration with half-filled 4f- levels which are relatively more stable. Gadolinium has the f7 d1 configuration, consistent with our expectation of a stabilized half-filled f shell.
The La and Lu elements above have no partially filled 4f orbital in their ground state. However, they are considered lanthanoids because of their properties close to rare earth metals. Cerium, where the increase in effective nuclear charge after Lanthanum is insufficient to stabilize the 4f2 5d0 configuration compared to 4f1 5d1. The nuclear charge is therefore not enough to contract the 4f orbitals which lower their energy well below the 5d.