Chemical Thermodynamics: Extensive properties
A property of the system is any characteristic specific to it. Physical properties are often referred to as observables. They are not modal properties. Properties can either be basic or derived. Basic property is directly measured while derive property is indirectly calculated from the basic ones. Properties can also be explained with respect to the directionality of their own nature. For example, isotropic properties never make differences with the way of observation, and anisotropic properties always have spatial variance.
Since the importance of an extensive property goes straightly with the mass, if a calculation of matter in a given state is parted into two equal pieces, each piece will have the same importance of the concentrated property as the real one and 50
Extensive properties are those whose values depend on the size or the extent of a system. Extensive properties are additive for subsystems. The system could be divided into any number of subsystems and the value of the property for the system would be the sum of the property for each subsystem.
Examples of extensive properties include
- energy
- entropy
- Gibbs energy
- length
- mass
- particle number
- momentum
- number of moles
- volume
- magnetic moment
- electrical charge
- weight
A molar property (xm) is the value of an extensive property x of the system for 1 mole of a substance. If ‘n’ is the amount of matter then xm = x/n is independent of the amount of matter.
Figure: Examples of extensive properties
The ratio of any 2 concentrated properties is an intensive property. The most common example is density, which is the ratio of mass and volume (both extensive) but is itself intensive since it does not change as the amount of substance changes.
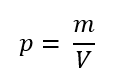
The difference between intensive and extensive properties is apparent to the difference between “quality” and “quantity”. As an example, the concentration of salt in seawater is the “quality”, while the 1 kg of gold is the “quantity”.
There are 4 properties in any thermodynamic system, 2 are concentrated and two are extensive.
If the set of parameters are intensive properties {a i} and another set, are extensive properties {Aj}, then the function is an intensive property if for all
![]()
It goes, viz, the ratio of 2 extensive properties is a concentrated property – density (intensive) is as mass (extensive) parted by volume (extensive).
Although not true for all physical properties, some properties have corresponding extensive and intensive analogues, many of which are thermodynamic properties. Examples of such extensive thermodynamic properties, that are dependent on the size of the thermodynamic system in question, include volume, internal energy, enthalpy, entropy, Gibbs free energy, Helmholtz free power, and heat ability (in the mean of thermal mass).