Ionic Equilibrium: Hydrolysis of salts
Salts are electrolytes that are produced when an acid reacts with a base. Because of the dissociation, the ions involved can cause water molecules to dissociate. This process of the dissociation of water molecules into hydronium (H3O+) and hydroxide (OH–) is called Hydrolysis. Hydrolysis is a term applied to reactions of ions in water that change the pH from 7.
In a salt solution, the anion (negative ion) is the conjugate base of an acid. Therefore, the addition of a proton indicates towards the acid formed and its strength. A strong acid makes a weak conjugate base and a weak acid makes a strong conjugate base. A weak conjugate base has a negligible tendency to cause the hydrolysis of water, thus limiting the production of OH- ions in solution. Here, no effect on the pH can be observed. However, a stronger conjugate base will have larger tendencies to hydrolyze water. The H+ ions react with the anion in solution, leaving behind OH- ions. This causes the pH to increase and turning the solution to be alkaline.
In general, when some salts ionize, they produce acidic solutions as they contain positive ions that release protons to water. Other salts ionize to produce basic solutions as they contain negative ions that attract protons from water. In salt hydrolysis, the cations of the dissociated salt remove the hydrogen ions. Alternatively, the anions of a dissociated salt donate hydrogen ions to water.
Consider the following reaction:
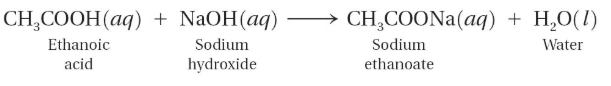
Sodium ethanoate is completely dissociated into ions in water. Since sodium ions are weak conjugate acid, they do not interact with the water ions.
![]()
However, the ethanoate ions come from a weak acid. This means ethanoate ions are strong conjugate acid that can interact with the hydrogen ions from water.
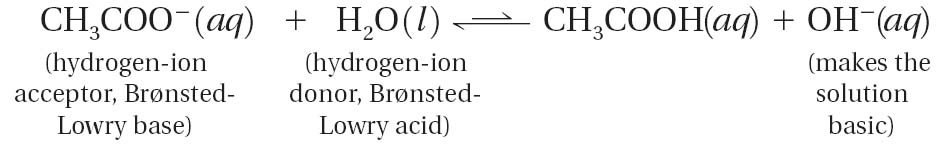
The equilibrium lies to the side of the ethanoic acid (to the right), removing the hydrogen ions from the solution. As [H+] decreases the pH rises. This means that the solution of sodium ethanoate has a pH greater than 7. This means that sodium ethanoate is a basic salt.
Consider another example of an ammonium chloride solution
Ammonium chloride completely dissociates into ions in solution to give
NH4Cl (aq)↔NH4+(aq) + Cl–(aq)
The ammonium ions interact with the hydroxide ions and remove them from water to shift the equilibrium to the right.
NH4+(aq)+ OH–(aq)↔NH3(aq)+ H2O(aq)
This increases the concentration of hydrogen ions (as [H+] x [OH–] is constant) increasing the acidity of the solution, thereby decreasing the pH. This means that ammonium chloride is an acidic salt.
To determine whether a salt solution is acidic or basic, remember that when strong acid reacts with a strong base, it gives a neutral salt. When a strong acid reacts with a weak base, it gives an acidic salt. And, when a weak acid reacts with a strong base, it gives a basic salt. If the salt is formed from a weak acid and weak base then its hydrolysis is deduced by analyzing the relative Ka and Kb values
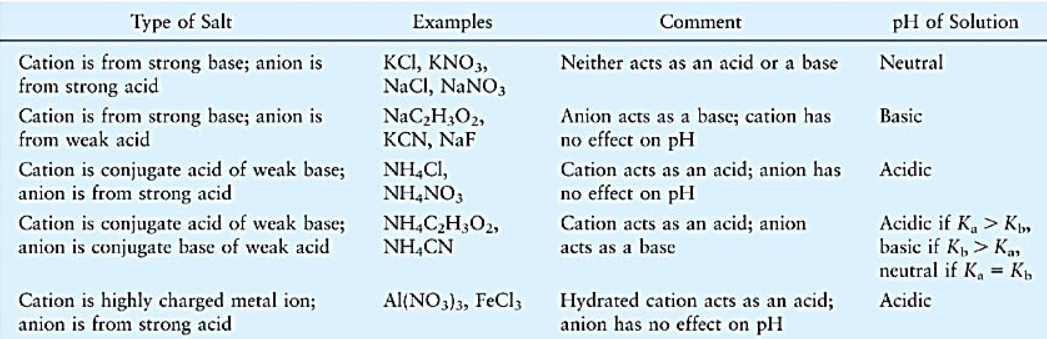
Fig: Acid-base properties of aqueous solutions of various types of salts.