p-Block Elements: Coordination compounds
Importance of coordination compounds (in qualitative inclusion, extraction of metals and biological system)
In chemistry, a coordination or metal complex compounds consists of an atom or ion which is usually a metallic ion that is surrounding by multiple molecules or anions known called ligands or complexing agents. Many metal-containing compounds consist of coordination complexes. A simple ionic reaction between coordination complexes and ligands requires electron transfers in solution. There are two different mechanisms of electron transfer redox reactions: the inner sphere or outer-sphere electron transfer. In electron transfer, an electron moves from one atom to another, changing the charge on each but leaving the net charge of the system the same.
In Chemical analysis, coordination compounds are used in both qualitative and quantitative analysis.
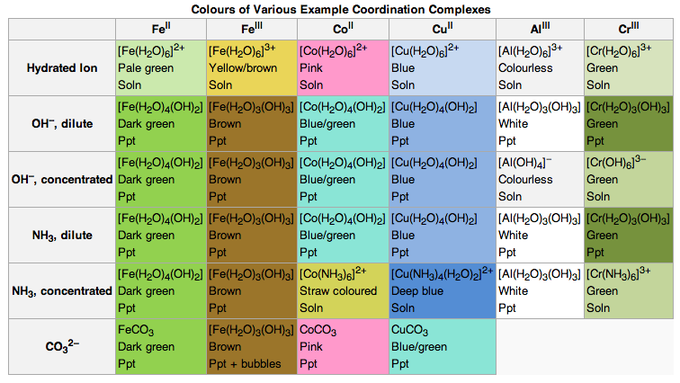
Qualitative analysis for metal ions
- Blue = CoSCN+
- Red = FeSCN2+
- Ni2+ and Pd2+ form insoluble coloured precipitates with dimethylglyoxime.
The coordination chemistry of the metals is also important for understanding the solubility and reactivity of mineral ores when they are extracted and refined into precious metal.
Examples of coordinate compounds in extraction by complex formation includes
-
- Silver and gold as cyanide complexes
- Nickel as Ni(CO)4(g)
Chelation is the formation or presence of two or more separate coordinate bonds that occur between a polydentate ligand and a single central atom.
Chelating agents are used in heavy metal poisoning
-
- EDTA for Pb poisoning
Commercial colouring agents in Inks, blueprinting, cosmetics, paints
-
- Prussian blue = mixture of hexacyano complexes of Fe(II) and Fe(III)
- Prussian blue = mixture of hexacyano complexes of Fe(II) and Fe(III)
Fig: Chelation therapy removes heavy metals from the body in the following steps. 1. An iron chemical compound is injected into the body. 2.. This compound renders the unwanted metal to a chemically inert compound by binding to it. 3. This allows the meatal to safely pass through the body without causing any further harm.
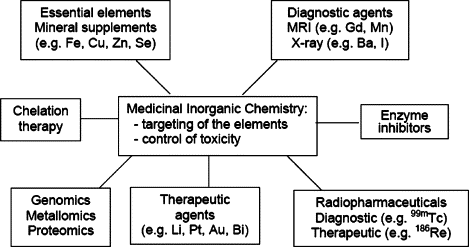
Chelation therapy incorporates chelating agents for detoxifying poisonous metal agents, such as mercury, arsenic, and lead. The chelating agents convert the metals to a chemically inert form that can be excreted without further interaction with the body.
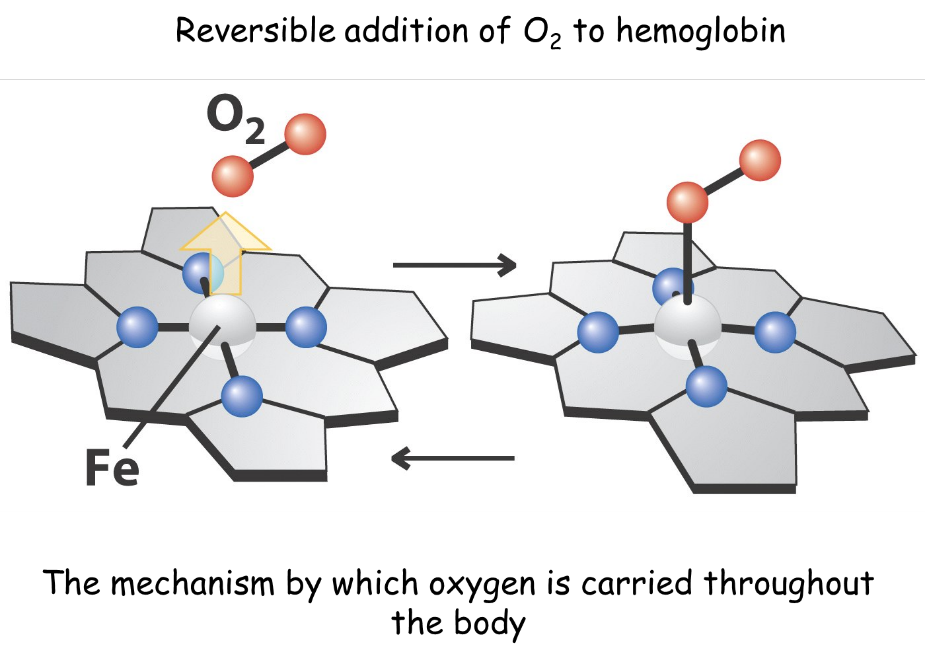
Coordination complexes are found in many biomolecules, especially as essential ingredients for the active site of enzymes. Some critical enzymes in our cells are metalloproteins, giant biomolecules which contain a metal atom. These metalloproteins are responsible for important life processes such as respiration and immunity. Porphines and hemes are important molecules in living systems. These planar molecules have a “hole” in the centre which to which a metal can coordinate. Haemoglobin is a metalloprotein which contains an iron atom and transports O2 throughout living systems. Vitamin B12,(Co[C62H88N13O14P])CN, which prevents pernicious anaemia, contains a Co atom which gives the vitamin a red colour. This vitamin is required for numerous crucial biological processes, including the production of red blood cells
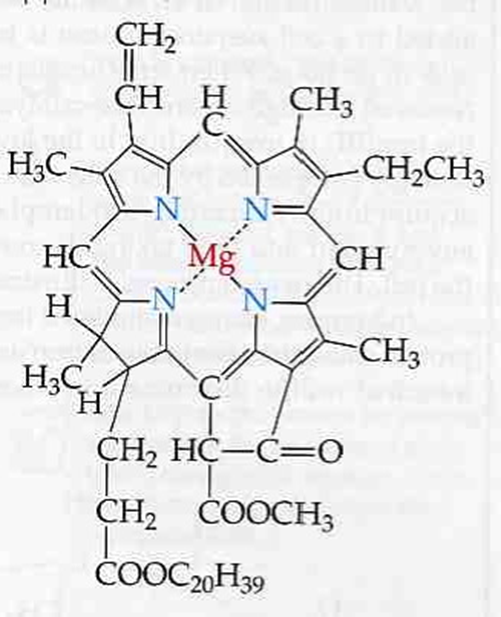
Likewise, Chlorophyll, (C55H72N4O5Mg) is a very important porphine that converts solar photons into food energy.