d and f Block Elements
Interstitial compounds
Transition metals are capable of forming complex non-stoichiometry compounds. These are compounds with indefinite structure and proportions. For example, Fe0.94O has a structure that is mostly due to the variable valency of transition elements. Non-stoichiometry is a result of defects in the solid structures and variable oxidation state of transition metals.
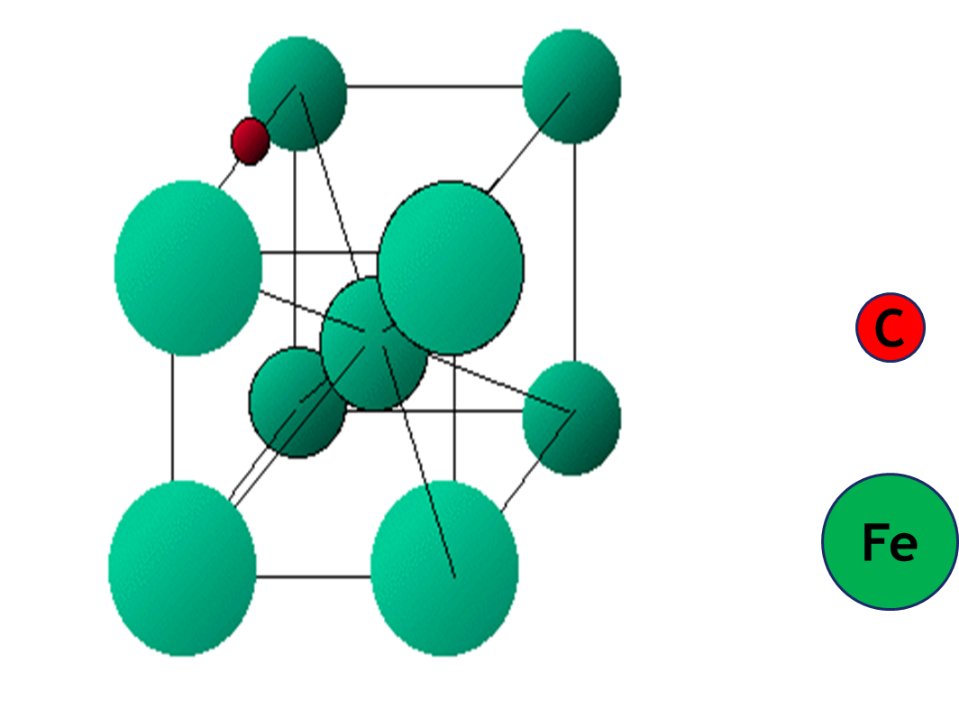
The transition elements form a large variety of interstitial compounds. In these compounds, small atoms such as hydrogen, carbon, boron and nitrogen occupy the empty spaces in their lattices. The small atoms enter into the voids or interstitial sites between the packed atoms of the crystalline metal. They are usually non-stoichiometric and are neither typically ionic nor covalent, for example, TiC, Mn4N, Fe3H, VH0.56 and TiH1.7, etc. The formulas of non-stoichiometric compounds do not correspond to any normal oxidation state of the metal. Due to the characteristic properties of their composition, these compounds are referred to as interstitial compounds. These compounds are hard and have higher melting points than those of pure metals. They retain metallic conductivity and are chemically inert. The presence of small atoms results in a decrease in malleability and ductility of the metals but increases their tensile strength.
The interstitial compounds can also exhibit semi conductivity, fluorescence and behave as heterogeneous catalyst. The catalytic activity of d-block elements and their compounds is associated with their variable oxidation states and their capability of forming interstitial compounds which can absorb and activate the reacting species.
Elements of the 3d-transition series can form interstitial compounds, e.g., Ti2C, V2C, ScN, TiN, Fe4N etc. These compounds have the properties of alloys being hard and good conductors etc. These elements also form non-stoichiometric compounds. For example, titanium forms TiOx (x=0.65 – 1.25 and 1.998 – 2.000); vanadium forms VOx(x= 0.79 -1.29); manganese forms MnxO (x= 0.848 – 1.00); iron form FexO (x = 0.833 – 0.957),etc. These compounds have a variable composition and are formed due to the variability of oxidation states and solid defects. Sometimes the interstitial and nonstoichiometric compounds are the same.
| Interstitial compound | Non stoichiometric compound |
| Vacant space present in a crystal lattice is known as a void or an interstitial site. When small elements like C, H, N, O occupy interstitial sits, the resultant compound is called interstitial compound. | Compounds that do not have elements in the exact ratio are called non-stoichiometric compounds. The variability of oxidation state allows transition metals to switch between states and thereby creating a compound by trapping other elements in different ways. |
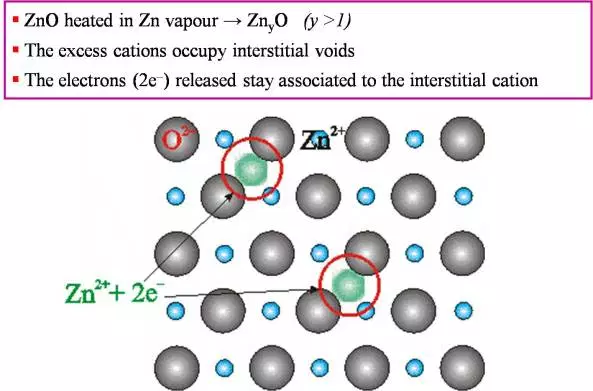
Fig 1: ZnO heated in Zn –> Zn2O (y>1). The excess cations occupy interstitial voids. The electrons 2e- released stay associated with the interstitial cation. |
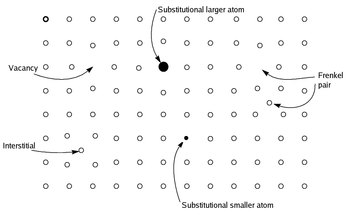
Fig 2: Various types of non-stoichiometric compounds: Substitutional larger atom, Frankel pair, Vacancy, Interstitial, Substitutional smaller atom. |