d and f Block Elements
Ionization enthalpy
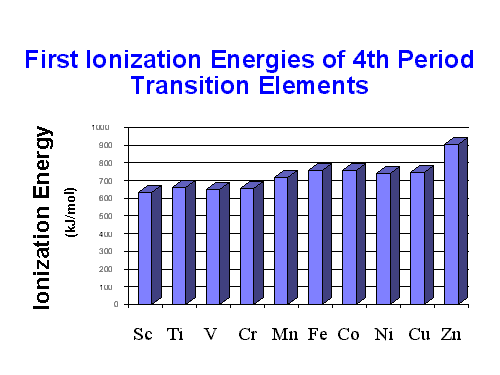
The first IE of the transition metals gradually increases as we move across a series. It is higher than those of s-block elements but lower than p-block elements. Consider a transition metal where electrons are initially removed from the 4s sub-level and then the 3d sub-level. between sublevels, only a small difference in energy is observed. When both sub-levels are empty, the 4s sub-level is slightly lower in energy and is filled first. In a particular transition series, ionization energy essentially increases gradually as we move across a series but this increase is not appreciable as we move from metallic to non-metallic character.
The increase in ionization energy is owed to the increase in nuclear charge. An increase in nuclear charge is to some extent are balanced by the increase in screening or shielding effect. This means that the increase in ionization energy along the period of d-block elements is minimal. Along the period, especially in the first transition metal series, the addition of the extra electron in the (n-1) d level happens. This electron upholds a screening effect and shields with the outer ns electrons from the nucleus. Hence, the effect of nuclear charge (effective nuclear charge) on outer ns electrons is slightly less than the actual nuclear charge. Increasing nuclear charge and the shielding effect created because of the expansion of (n-1)d orbital oppose each other. As a result of these counter effects, the ionization potentials increase is minimal on moving in a period of the first transition series
Metals from scandium to zinc show a small change in the values of the first and second ionization energies because of the shielding effect. The build-up of electrons in the immediately underlying d-subshells that efficiently shields the 4s electrons from the nucleus and minimizing the increase in effective nuclear charge Zeff from element to element. Alternatively, the increases in 3rd and 4th ionization enthalpy values are more rapid. However, the trends in these values show the usual discontinuity halfway along with the series. The reason is that the five d electrons are all unpaired, in singly occupied orbitals. When the sixth and subsequent electrons enter, the electrons have to share the already occupied orbitals resulting in inter-electron repulsions, which would require less energy to remove an electron. Hence, the third ionization energy curve for the last five elements is identical in shape to the curve for the first five elements but displaced upwards by 580 kJ mol-1.
IE2 :V< Cr > Mn & Ni < Cu > Zn
IE3 : Fe << Mn
Due to an increase in nuclear charge which accompanies the filling of the inner d- orbitals, There is an increase in ionization enthalpy along with each series of the transition elements from left to right. However, many small variations occur due to the removal of an electron from a completely filled or a half-filled subshell which requires a relatively large amount of energy. Such is the case of zinc for first ionization energy