The ionization enthalpy of the elements is the amount of energy that is required by an isolated gaseous atom for losing an electron in its ground state. When atoms lose the electrons then it causes the formation of positive ions (cations). The first ionization energy of an element is the energy required by the atom for the formation of A+ ions. In the same way, second ionization energy is the energy required to remove the second electron from its valance shell.
A specific amount of energy is required to remove the electrons from atoms. So, chemical elements always have positive ionization enthalpies. As compared to the first outer electron the second most outer electron will always be more attracted to the nucleus. So, second ionization energy is always greater than the first ionization energy. Similarly, the third ionization enthalpy will be more than the second one. Usually, this energy is expressed as KJ/mol.
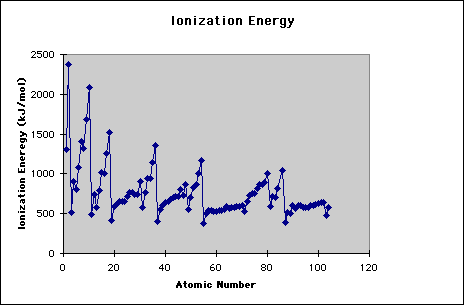
Understanding the ionization enthalpy is important to study the chemical reactions and to understand the behavior that either various atoms make ionic or covalent bonds with each other. The elements which are placed close to each other in the periodic table do not have much difference in their ionization enthalpies and make covalent or polar covalent bonds. Ionization enthalpies are greatly dependent on the atomic radius.
Mainly the ionization enthalpy depends on the two factors.
• The attraction forces between nucleus and electrons.
• The force of repulsion between the electrons.
Trends of Ionization Enthalpy in Group I
By moving down in the group, the first ionization enthalpy of the elements is decreased while the atomic number and number of shells are increased. The electrons in the outermost shell are quite away from the nucleus so they can be easily removed. Ionization enthalpy is also decreased due to the shielding effect. Alkali earth metals have the lowest ionization enthalpies, especially when they are compared to the halogens. The ionization energy of lithium is 520, sodium 496, potassium 419, rubidium 408, cesium 376, and francium 398.
Trends of Ionization Enthalpy in Group II
Group II elements of the periodic table have the higher ionization enthalpies and more energy is required to remove the electrons from the outer shells of the atoms. The ionization energy for beryllium is 899.5, 737.7 for magnesium, 589.8 for calcium, 549.5 for strontium, 502.9 for barium and 509.3 for radium. Alkaline earth metals have high ionization enthalpy than the alkali metals as the tendency of alkali metals to lose electrons is higher.
As with the alkali metals, the ionic and atomic radii of the alkaline earth metals smoothly increase from beryllium to the barium so a decreasing trend is observed for the ionization enthalpies. The ionization enthalpy of the first metal in this group is always greater than the other metals in this group. As they have the low first and second ionization energies so these elements exclusively make ionic compounds containing M2+ ions. However, the lightest element beryllium that has small size and high ionization energy largely form the covalent compounds.