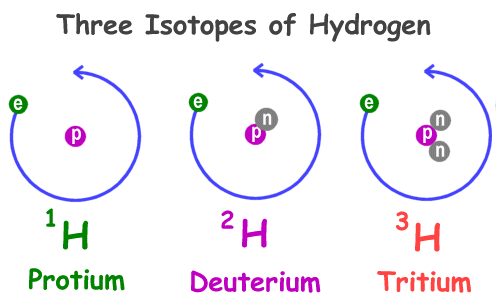
Naturally, there are three isotopes of hydrogen and which are denoted as 1H protium, 2H deuterium, and 3H tritium. Other isotopes have been synthesized in the laboratory ranging from 4H to the 7H and they are highly unstable and are not observed in nature.
Protium 1H
Among the naturally occurring isotopes of hydrogen 1H is the most abundant one and its occurrence is about 99.98%. This isotope is called protium and its atomic mass is 1.00782504. The nucleus of this isotope has only one proton so the descriptive, but rarely used formal name protium is given to this isotope. It is also known as the ordinary hydrogen
Deuterium 2H
2H is another isotope of hydrogen that is stable and its name is deuterium and, in its nucleus, it has one neutron and one proton. It is thought that all the deuterium was essentially produced at the time of big bang and since that time it is endured. It is the second most abundant isotope of hydrogen and it makes 0.0026 to 0.0184% of the hydrogen that is naturally found on the earth. The atomic mass of the deuterium is 2.01410178. Its nucleus is two times heavier than the nucleus of protium so it is also called heavy hydrogen. Deuterium was discovered by the American Chemist Harold C. Urey in 1931.
This isotope is not radioactive and it does not represent the significant toxicity hazards. Water enriched in the deuterium molecules instead of the normal hydrogen is called heavy water. Heavy water is used as a coolant and a neutron moderator for the nuclear reactors. In the chemical experiments, deuterium and its compounds are being used as a non-radioactive label and are also used in the solvents for 1H-NMR spectroscopy. Deuterium is also used as a potential fuel for the commercial nuclear fusion process.
Tritium 3H
3H is also an isotope of hydrogen called tritium and, in its nucleus, it has two neutrons and one proton. This isotope is radioactive and it decays into helium-3 through the process of beta decay with a half-life of about 12.32 years. Sufficiently, it is radioactive and it is used in luminous paint and making it useful in things such as watches where glass moderates the amount of radiation that is getting out. Its atomic mass is 3.0160492.
A small amount of the tritium does occur naturally due to the interaction of atmospheric gases with the cosmic rays. During the tests of nuclear weapons, tritium has been released. Tritium is widely used in the nuclear fusion reactions, as a tracer in the isotope geochemistry, and it is specialized for the self-powered lighting devices. It has been used in the biological and chemical labeling experiments as the radiolabel.
Hydrogen is the only element that has different names for its isotopes which are commonly used nowadays. Earlier, during the study of radioactivity, names were given to the various radioactive isotopes, but now those names are not used except for the tritium and the deuterium.