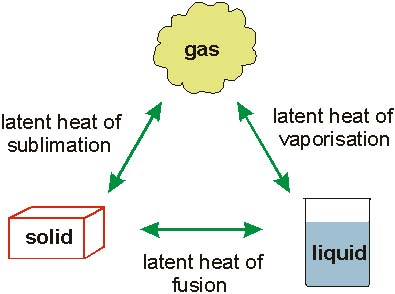
Latent heat is the energy released or absorbed by the material or substance during the changes in its physical state, that occurs without causing any changes in its temperature. The latent heat which is associated with the freezing of liquid or melting of the solid is known as the heat of fusion. Whereas, the latent heat that is associated with the condensing of vapor, or vaporizing of the liquid is known as the heat of vaporization.
Latent Heat is Used for Breaking the Bonds
Normally, the latent heat is expressed as the amount of heat in the units of calories or joules per unit mass or mole of the substance that is changing the state. During the changes in the state of any substance, there may be no increase in the temperature at that time, where the change of the state is occurring, even though the heat is being added. All the added heat energy is used for breaking the bonds, which is essential for completing the change of the state.
Latent Heat of Melting
The latent heat rises from the work, which is required to overcome the forces of attraction which hold the atoms or molecules in the materials. By the forces of attraction among the individual atoms, the regular structure of the crystalline solid is maintained. With the increase in the temperature, the motion of the particles becomes increasingly vibrant and at the melting point, the forces of attraction are not sufficient for the maintenance of the stability of the crystal lattice. However, the additional heat in the terms of latent heat of fusion must have to be added to accomplish the transition to the more disordered liquid state.
Latent Heat of Vaporization
The liquid is different from the gas as the forces between the gasses are even much weaker than the forces between the water molecules. In the water, the forces of attraction are sufficient for maintaining the long-range order that is responsible for the degree of cohesion in the water molecules. With the further increase in the temperature, the second transition point that is the boiling point is reached, where the long-range order starts becoming unstable. Resultantly, the independent motion of the particles is observed and the liquid is changed to the gaseous state. Here the latent heat of vaporization, in terms of additional heat should be added for breaking the longer range of the order of the liquids to accomplish the transition to the greatly disordered gaseous state.