Lewis Structure is defined as an easy and simple representation of valence shell electrons or valence electrons in a given molecule. Used in demonstrating how electrons are classified and arranged throughout the individual atoms in a given molecule. Electrons are displayed in the form of “dots” or for bonding any electron; it’s showcased by lines between two atoms.
The target is to achieve the “best” of the electronic configuration, which means, it should satisfy the formal changes the Octet Rule.
Important Thing to Know
Lewis Structure doesn’t make an effort to describe molecule geometry, how the formation of a bond is done, or how you can share the electrons between atoms. This is the easiest and the most definite theory related to electronic structure.
How you can draw Lewis Diagrams?
A blueprint of knowing how to make the “best” Lewis structure
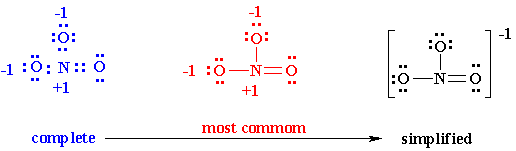
Let’s take NO3– example
1. Find out the total number of electrons in the outermost shell or valence electrons present in a molecule
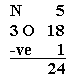
2. Draw a diagram for a molecule, connecting all the atoms using only one “single or lone bonds”. In molecules which are simple in nature, atoms are usually placed in the center with most convenient spots for bonding. The count of bonding sites is figured out, taking everything in kind regarding the atom’s ability to extend its octet and the total of valence electrons. As you tend to become more desirable, you’ll be able to examine that specific atoms’ groups favor to bond in a group or bond together in a particular manner.
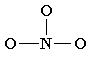
3. In NO3–, 6 out of 24 valence electrons, were needed to design the skeleton. Now look at the rest of the 18 electrons and fix them at a place in order to occupy whole of the octet with a lot of atoms as per their possibility (firstly, begin with extreme electronegative atoms and then proceed further to numerous electropositive atoms.
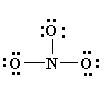
4. Now see that the octets of all atoms (like in eg. N, O) are filled? If they are not, then fill the rest of the octets by building multiple bonds (create a single pair of electrons, which is located on an additional electronegative atom, in an electron pair bonding which is shared with an atom i.e. electron deficient (not having that much electrons which are required)
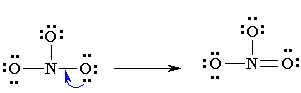
5. Analyze that you acquire the lowest Formal Charges (A formal charge refers to a charge allocated to the atom in a given molecule, presuming that electrons are equally shared in between the atoms, existing in each and every chemical bond, disregarding the relative electrolysis. It’s attainable for whole atoms, without opposing “Octet Rule”. (valence electrons) – (1/2 bonding electron) – (single or lone electrons).
Formal Charge in Lewis Structure:
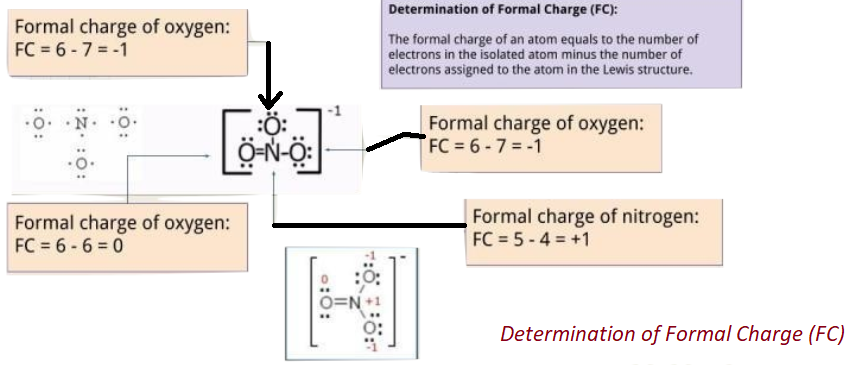
Important To Note: Formal Charges plays an important role in Lewis Structure as no diagram of Lewis structure is meant to be complete without the presence of Formal Charges. Lewis Diagrams are needed to analyze the mechanism to know that which molecule part is electron rich (-) and which is electron deficient (+) and it’s vital to know this.
It is best to gain 0 “formal charge” to keep a possible considerable amount of atoms in a given structure.
The formal charge is minimized in general if 1- formal charge is placed next to 1+ formal charge by including a “lone or single pair of electrons”, placed on an atom with a charge of 1- becoming an electron pair bonding which is shared with an atom, containing formal charge of 1+ (this can be envisioned alike the multiple bond formation as given above)
Attention: Octet can be extended to reduce the formal charge, but only for second-row atoms present in the periodic table (where n = 3 or more).
6. You might notice the finest Lewis Diagram (the diagram with “lowest formal charges” and all the satisfying octets) has been given in as many different ways. For the molecule of NO3-, 3 different lewis diagrams are mentioned below. The complete Lewis structure starts from left up to the easiest one.