p-Block Elements: Coordination compounds
Magnetic properties and shapes
Diamagnetism and paramagnetism are properties of substances, which can be observed in the presence of an externally applied magnetic field by a weak repulsion or attraction of the substance from/into the magnetic field.
Bohr magneton μB is the smallest quantity of a magnetic moment
(M: a mass of the electron, e: charge of an electron: h: Plank constant; c: speed of light)
The magnitude of the crystal field splitting also helps in understanding the magnetic properties of a complex ion. The electron configuration of a transition metal is a balance between
- Energy to promote an electron to a higher energy d orbital – related to the magnitude of ∆
- The stability gained by the maximum number of unpaired spins
Small values of ∆ favour maximum number of unpaired spin for high spin complexes. For example, F– is low on spectrochemical series
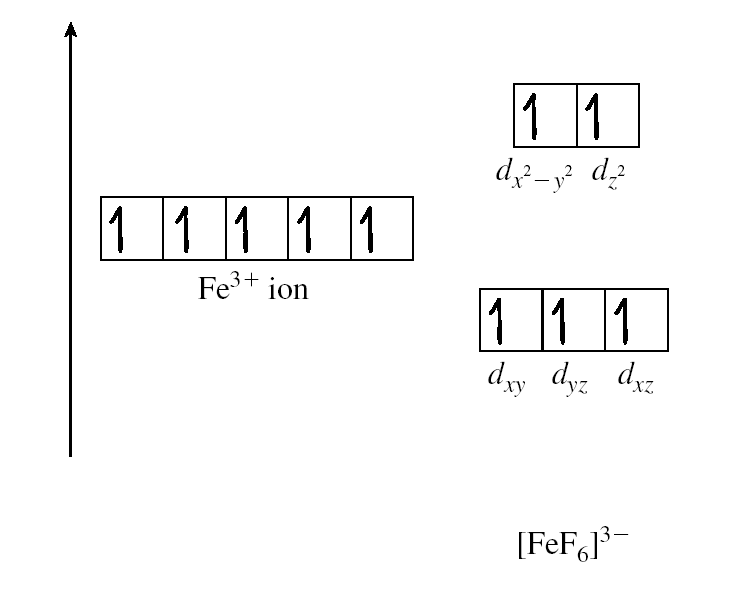
Large values of ∆ are unfavourable for promotion. They produce low spin complexes such as CN–which is high on the spectrochemical series
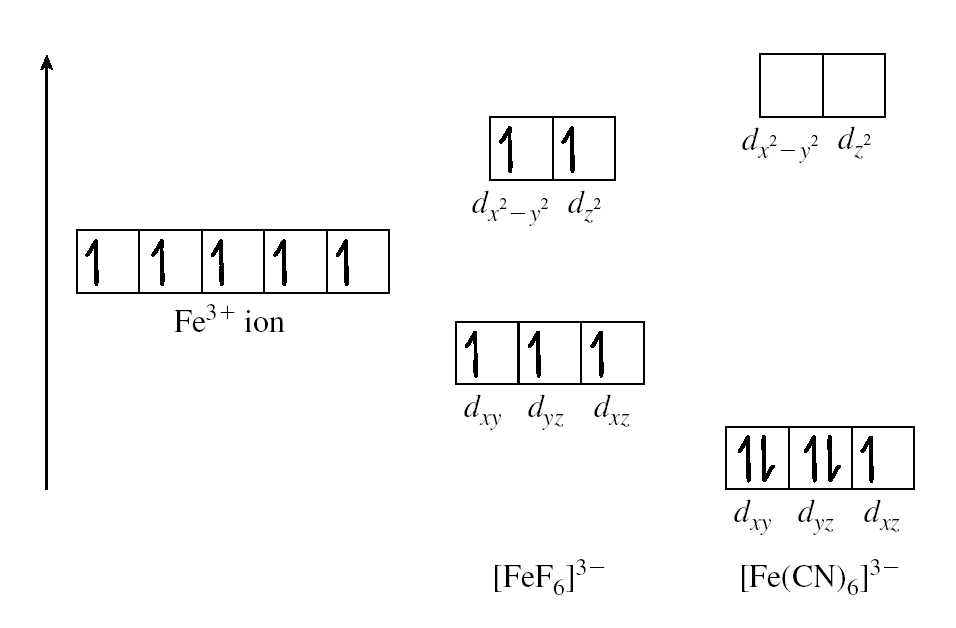
The attractive force towards a magnetic field (H) can be explained by Magnetic moment ():
≈ [N(N + 2)]1/2B
where N = number of unpaired electrons
| N | /B |
| 1 | 1.73 |
| 2 | 2.83 |
| 3 | 3.87 |
| 4 | 4.90 |
| 5 | 5.92 |
This is the paramagnetic contribution from unpaired e spin only. It does not consider both spin-orbit coupling and diamagnetic contributions
eg: [Mn(NCS)6]4 experimental /B = 6.06, the magnetic moment shows that Mn(II) is d5 high spin complex.
The strength of the crystal field determines the electron configuration of the metal ion with split d orbitals. The fourth and fifth electrons will go into the higher energy if the field is weak and the energy gap is small, leading to unpaired electrons and a paramagnetic complex. The fourth through sixth electrons will pair the electrons in the dxy, dyz, and dxz if the field is strong and the energy gap is large, leading to paired electrons and a diamagnetic complex. Only electron configurations d4, d5, d6, or d7 can show any sign of magnetism with either low or high spin.
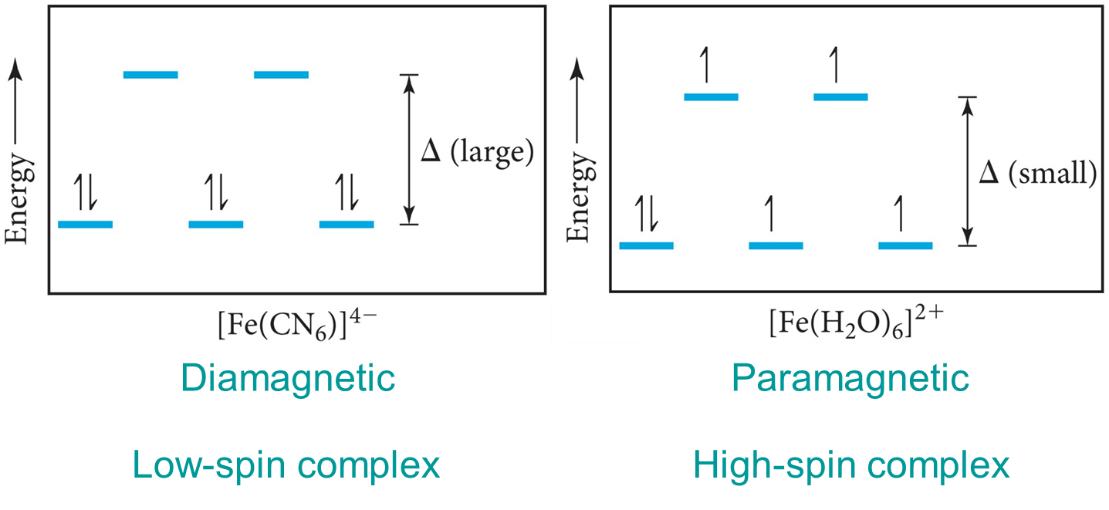
Weak-field ligands yield high-spin configurations while strong-field ligands yield low-spin configurations.
Fig : Weak field ligands.
Fig : strong field ligands.
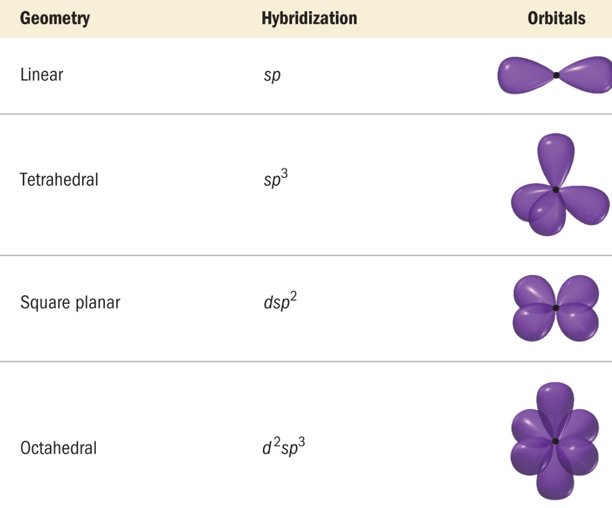
The structures of complexes are divided into following hybrid orbitals owing to the position of electrons in the orbitals:
d2sp3 octahedral, dsp3 trigonal bipyramid, dsp2 square planar, sp3 tetrahedral
The ligands in an octahedral complex are in the same space as the lobes of the orbitals. The repulsions between electron pairs in the ligands and any potential electrons in the d orbitals result in an increase in the energies of these orbitals. The other d orbitals lie between the axes and have nodes directly on the axes, which results in less repulsion and lower energies for these three orbitals.
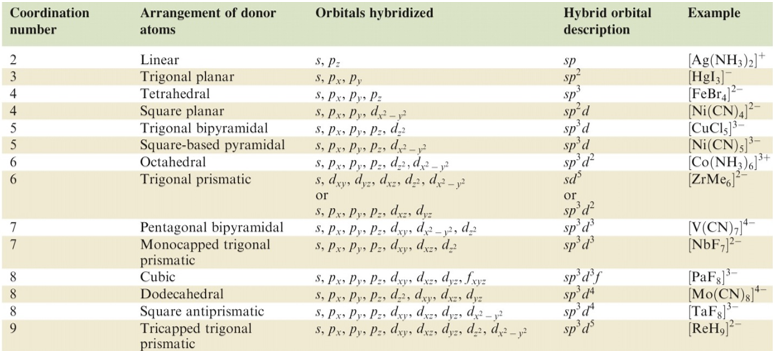
Figure 3: Hybridization schemes for the sigma bonding frameworks of geometrical configurations of ligands donor atoms.