Methods of Polymerization (Addition and Condensation)
Polymers are the giant molecules and they are formed when the monomers are linked together during the chemical reaction known as the polymerization. Generally, there are two types of polymerization reactions, addition reactions, and condensation reactions.
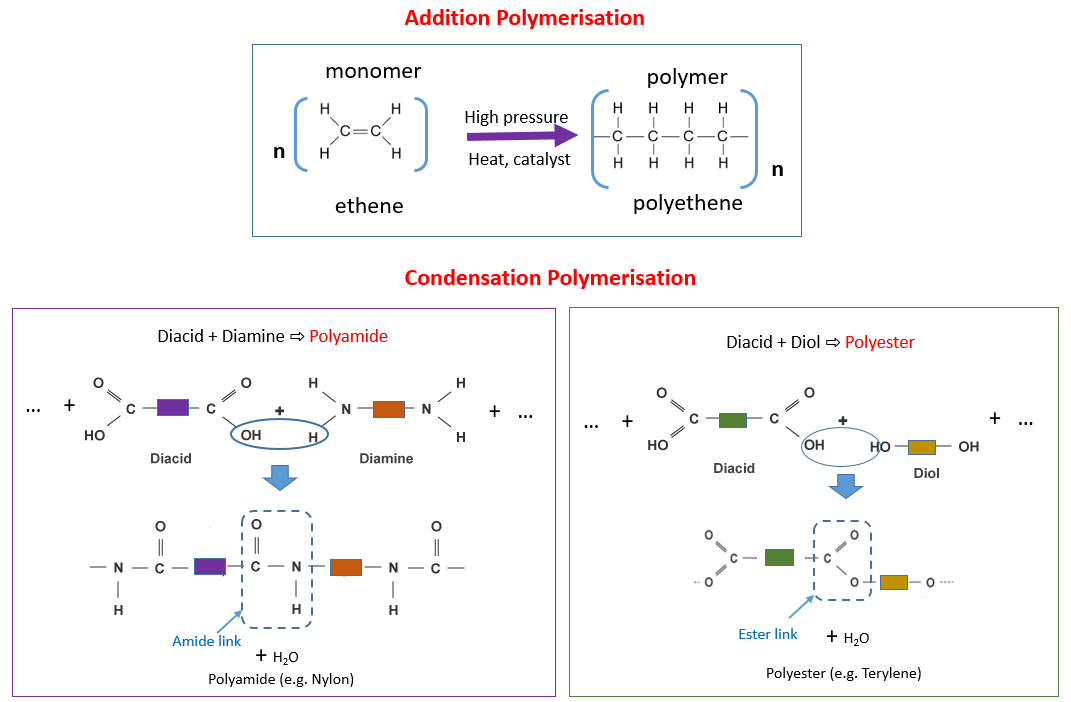
Addition Reactions
In the reactions of the addition polymerization, monomers are linked with the double bonds. The double bond of the one of the monomer breaks and is linked to the neighboring monomer. This process is continued and the monomers are formed. Some polymers may consist of thousands of the polymers. By the addition polymerization, the formation of a polymer is an example of a chain reaction. If this reaction is started once, then it keeps going y itself. The main steps of this reaction are initiation, propagation, and termination. Many processes are used to carry out the chain reaction of the addition polymerization and the details of the reactions greatly vary according to the used methods. Only the polymerization of alkenes is considered as the addition reaction. Addition polymerization reactions are faster than the condensation polymerization reactions.
Condensation Polymerization
During the condensation polymerization, monomers react together and cause the release of the small molecule in this process. Commonly this small molecule is hydrochloric acid or water. Countless of these polymers are bunched together and causes the formation of solid plastic. The polymers are held together by the weak forces and by the application of the heat, they can be easily disrupted. This plastic is easy to melt and is known as thermoplastic. Condensation polymerization causes the formation of condensation polymers.
There are many useful and important polymeric materials which cannot be made by the chain growth processes, including the radicals which are the reactive species. Instead, their formation is easy by the process by the transformations of the conventional functional groups of the polyfunctional reactants. Polyamide Nylon 66, and the polyester Dacron are common examples of the condensation polymers. Generally, these polymers grow by the bond formation of the carbon-heteroatom. These polymers can also be considered as the alternating copolymers, and the monomeric unit that is continuously repeated is defined as the combined moiety. Polypeptide chains, of the proteins, cellulose, are examples of naturally occurring condensation polymers. Generally, these reactions are lower in the molecular weight, and often they require the heat. The chain has the terminal functional group and it remains active and due to this reason; the groups of shorter chains are combined into the longer chains in the late stages of the polymerization reaction.