Amines are basically derivatives of the ammonia compound. These derivatives are formed by the replacement of the hydrogen atoms by the alkyl or the aryl groups. The amines are basic in nature. This is because the nitrogen atom has high electron density, as it has two valence electrons in its orbital. Also, the alkyl and the aryl groups further increase the density of electrons in the compound.
The amines are classified on the basis of the number of the alkyl and the aryl groups attached to the Nitrogen atom of amine. If one alkyl or aryl group is bonded then, the amine is known as 1° amine or it is also known as a primary amine. If two alkyl or aryl groups are attached, then it’s known as secondary amine or 2° amine. Similarly, goes for the 3° and 4° amines respectively.
Preparation of amines:
There are many methods of preparation of amines. They are as follows:
Alkylation of ammonia:
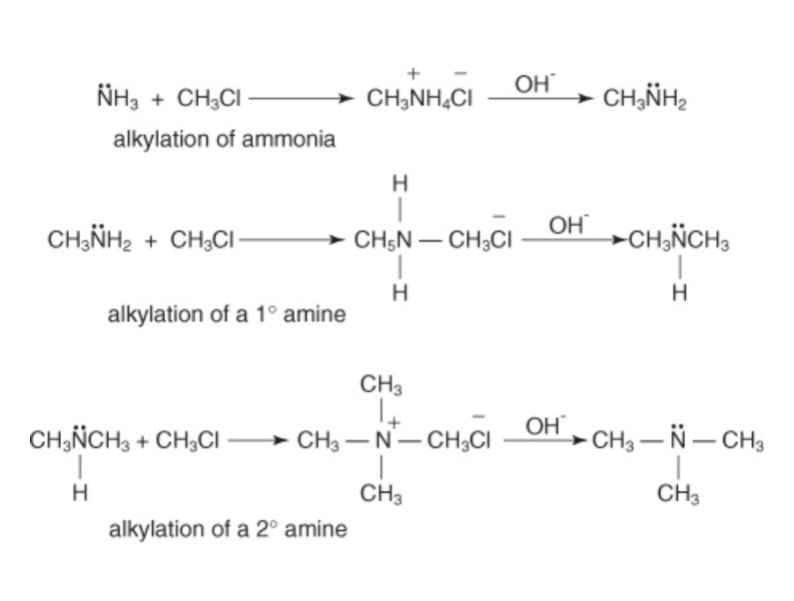
The amines react with alkyl halides to form the primary amines. The alkyl group from the alkyl halide has a positive charge and it is electron deficient. Hence, it attacks the electron rich ammonia. The reaction gives the final product as the primary amine. The primary amine on further reaction with alkyl halides leads to the formation of secondary amine. Later, the secondary amine again reacts with the alkyl halide and the tertiary amine is formed. Thus, in the reaction of ammonia with alkyl halides, a mixture of products of amines is formed.
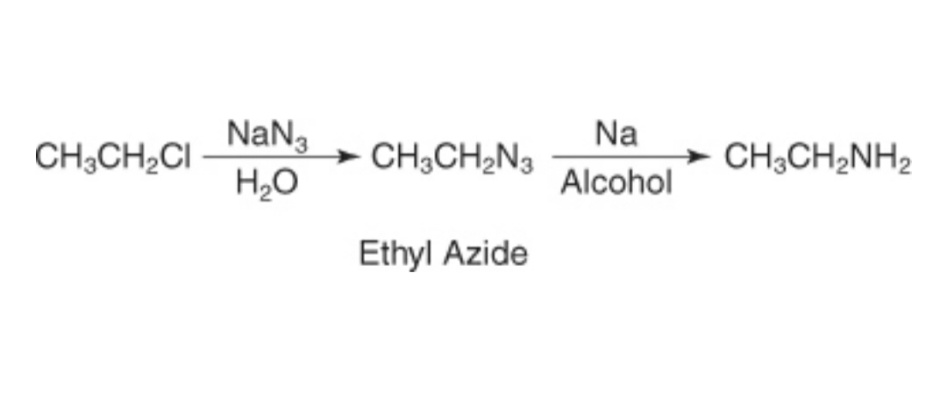
Reduction of alkyl azides
In the reduction of alkyl azides, the Gabriel synthesis process is used. Herein, the potassium phthalimide is reacted with an alkyl halide to form the product n-alkyl pthalamide. The n-alkyl pthalamide is further reacted with aqueous acid to give primary amines.
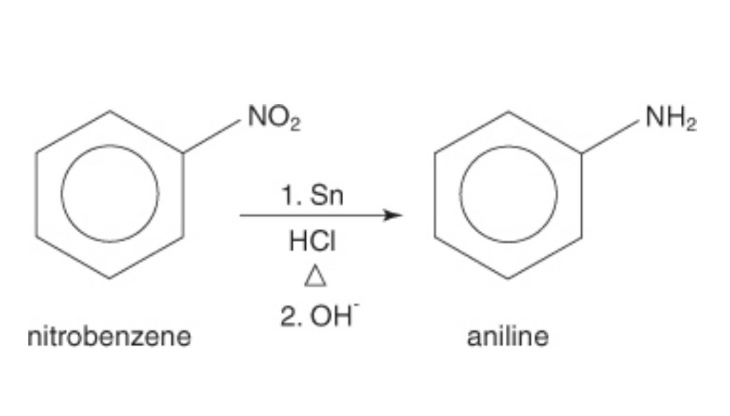
Reduction of nitro compounds
The anilines could be formed by the reduction of the nitro bonded compounds. One of the examples of the reduction of nitrophenyl. Here the nitro group attached to the phenyl is reduced with the help of proper reagents to a primary amine. The reducing agent used in the process is Sn + HCl, along with that little heat is supplied to make the reaction process faster.
Reduction of amides
The primary mines could be also prepared by the subsequent reduction of amides. The reducing agent used in the reduction of amides is LiAlH4. Further, the N substituted amide is reduced to secondary amine and the N, N substituted amine is reduced to a tertiary amine. This is the major process followed for the making of amines.