Carboxylic Acids are one of the imperative organic compounds found in a wide variety of living things. The Carboxylic Acid is part of the amino acids and amino acids are the building block of proteins. We all have heard about vinegar. The carbonyl group attached to hydroxyl in a compound represents the Carboxylic acid. Various methods can be used for the preparation of Carboxylic acids. They are as follows:
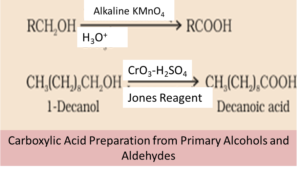
By using primary Alcohols and Aldehyde
We will notice that the primary alcohol gets oxidized to carboxylic acid when oxidizing agents are added such as potassium permanganate (KMnO4) in an acidic, alkaline or neutral medium. We can obtain carboxylic acids from aldehydes on using a mild oxidizing agent.
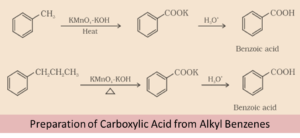
From Alkyl-benzenes
Aromatic carboxylic acid is obtained after vigorous oxidation of alkylbenzenes with chromic acid. Whole side chain is oxidized to a carbonyl group irrespective of the length of the chain. The oxidation of primary and secondary alkyl groups can be done in this manner while the tertiary group is unaffected.
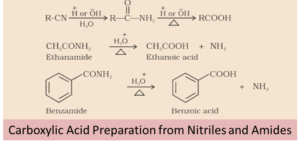
From Nitriles and Amides
Amides are prepared by the hydrolysis of nitriles and then it is converted to acids in the presence of catalysts (H+or OH-1). In order to stop the reaction at the amide stage, mild reaction conditions are used.
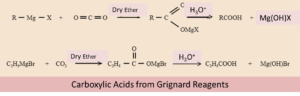
By using Grignard Reagents
When Grignard reagent is made to react with carbon dioxide, it forms salts of carboxylic acids. After some time it gives corresponding carboxylic acid post the acidification with any mineral acid. Both Grignard reagents as well as nitriles can be prepared from alkyl halides. For the conversion of alkyl halides into corresponding carboxylic acid, these methods are of great use.
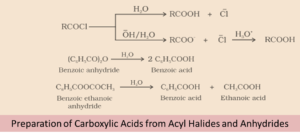
By Acyl Halides and Anhydrides
Carboxylic acid is produced by the hydrolysis of chloride with water. It is hydrolysed more readily with aqueous base and gives corresponding carboxylic ions. On further acidification it gives the carboxylic acid.
we can summarize
- First the hydrolysis of acid chlorides with water takes place to produce carboxylic acids
- Acid chlorides then undergo reaction with a base. Its further acidification leads to carboxylic acid
- When Hydrolysis of acid anhydrides takes place, it leads to formation of carboxylic acids.
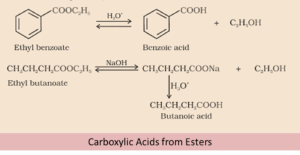
By using Esters
Carboxylic acid is obtained by acidic hydrolysis of esters. However, hydrolysis of the base produces carboxylates followed by acidification leads to the formation of corresponding carboxylic acids. Also the hydrolysis of esters is carried out with mineral acids or alkali in order to produce a carboxylic acid.