The chemical compounds containing arenes, where one or more hydrogen atoms bonded to an aromatic ring are replaced with halogens are called Haloarenes. It contains halogen atom(s) attached to sp 2 hybridised carbon atom(s) of an aryl group. The nature of C-X bond depends on both the halogen attached and the nature of carbon in the aromatic ring. Halogens are generally denoted by the alphabet “X”. Halogens are group 17 elements and have high electronegativity. These are namely, fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine(At). Fluorine has the highest electronegativity out of them. The elements in this group just need one more electron to complete the nearest noble gas configuration.
When compared with the electronegativity of halogen molecule, the carbon in haloarenes which is a 14th group element has lesser electronegativity. This is attributed to the fact that electronegativity increase across a period from left to right.
Salient features of the nature of C-X bond in haloarenes are:
- As the halogens are more electronegative than carbon, the C-X bond in haloarenes is polarized. Due to this high electronegativity of halogen, it attracts the electron cloud more towards itself. Due to this it gains slight negative charge. Similarly on the other hand, carbon obtains a slight positive charge.
- Halogens need only one electron to achieve their nearest noble gas configuration, therefore only one sigma bond is formed between one halogen and one carbon atom.
- The C-X bond length in haloarenes decreases from astatine to fluorine and bond dissociation strength increases. This is due to the increase in atomic size from fluorine to astatine.
- Dipole moment depends on the difference in electronegativity of carbon and halogens and as we know that the electronegativity of halogens decreases down the group, the dipole moment also decreases. An exception can be seen in case of C-Fand C-Cl dipole moments. Though the electronegativity of Cl is less than F, the dipole moment of a C-Cl bond is more than C-F.
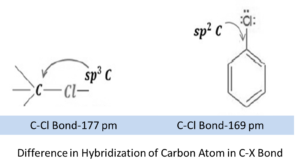
- The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of C—X bond more tightly than sp3 -hybridised carbon in haloalkane with less s-character. Thus, C—Cl bond length in haloarene is 169 pm whereas in haloalkane it is177pm.It is difficult to break a shorter bond than a longer bond. This makes haloarenes less reactive than haloalkanes in case of nucleophilic substitution reaction.