In organic chemistry, alcohols are one of the most important functional group. For the synthesis reactions, alcohols are a good source of reagents. Their identification is extremely important for looking at the NMR and IR spectra.
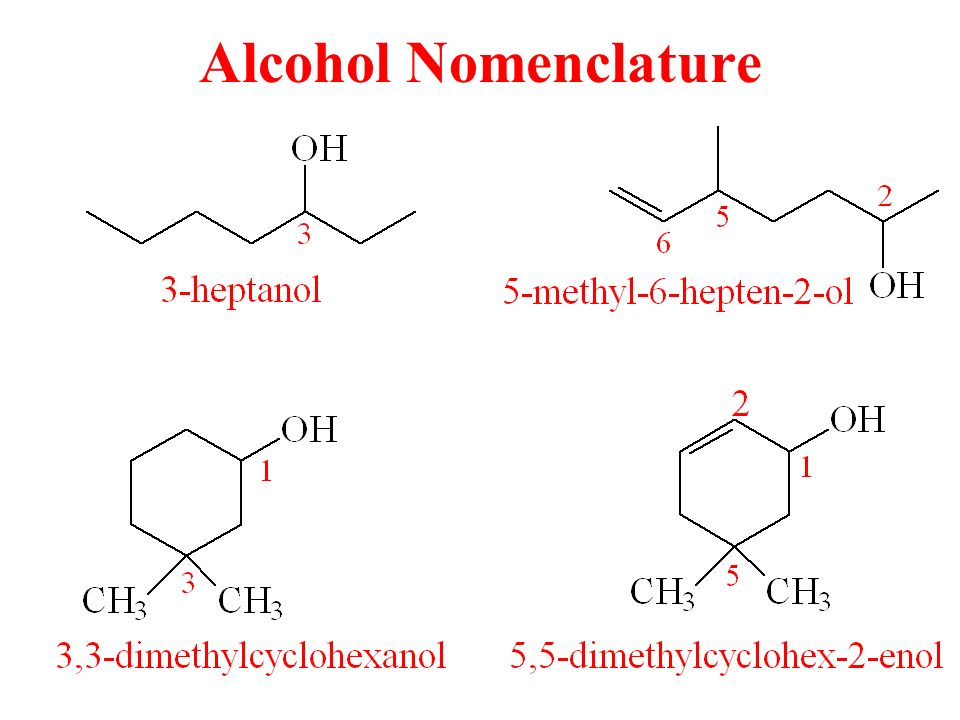
Alcohols that have one to four carbon atoms are called by their common names and the name alkyl group is followed by the word alcohol. According to the rules of the International Union of Pure and Applied Chemistry (IUPAC) for naming the alcohols the name of parent alkene is changed to –ol. Some of the basic rules for naming the IUPAC alcohols are as following.
- A longest continuous chain of the carbon atoms which is having the OH group is regarded as the parent compound and alkene having the same number of the carbon atoms. The numbering is started from the end that is nearest to the OH group.
- The number of carbon atom indicating the position of OH is prefixed to the name of the parent hydrocarbon and a suffix –ol is used to replace the ending of a parent alkene. In the cyclic alcohols, the carbon atom that is having the OH group is designated as C1. Substituents are numbered and named exactly in the same way as for the alkanes.
- If the same molecule has more than 1 hydroxyl group then the suffixes such as -diol and -triol are used. However, the ending of parent alkane is retained in these cases.
- Alcohols are named by removing the –ane of the parent compound and instead –ol is added. By using the group name hydroxy, the hydroxyl group can be named as the substituent.
For naming the salts of the alcohols, firstly, metal is named and then the alcohol ion is named such as alkoxide.
Phenolic compounds are similar to the alcohols but due to the aromatic ring, their chemical properties are much different. They can be easily named by using the nomenclature of the hydroxide group.
Alcohols have hydroxyl functional groups and they are organic molecules, where OH group is directly bonded to the carbon atom. Here the carbon on which OH group is attached is called carbinol carbon. However, it is a key factor for understanding the common classification of the alcohols that either it is primary, secondary, or tertiary. When there are three hydrogens and zerocarbon the situation is unique and it is methanol. The alcohols in which the hydroxyl group is attached to the aromatic rings is called the phenols.