p-Block Elements: Group 16
Occurrence
Elements in group 16 tend to bind with other metals to form common ores in the earth’s crust. Molecular oxygen is a gas at room temperature and 1 atm. It is colourless, odourless, and tasteless. It is also the most abundant element by mass inthe Earth’s crust and the human body.Oxygen makes up about 46.6% by weight of earth’s crust. Dry air contains about 21%oxygen by volume. Oxygen can form very strong ionic and covalent bonds with other elements.
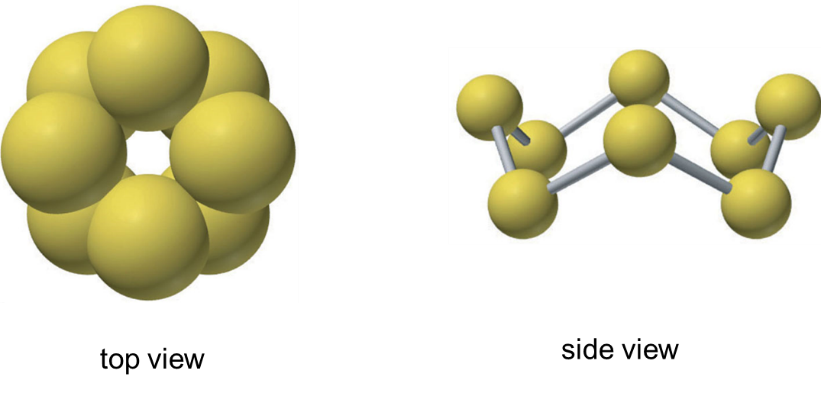
Fig 1: At room temperature, the sulfur molecule is a crown-shaped ring of eight atoms. Orthorhombic α-S8 is the most stable S allotrope and it consists of cyclo-S8.
Sulfur is a solid at room temperature and 1 atm pressure. It is usually yellow, tasteless, and nearly odourless. It is the sixteenth most abundant element in Earth’s crust. It exists naturally in a variety of forms, including elemental sulfur, sulfides, sulfates, and organosulfur compounds.Sulfur is unique in its ability to form a wide range of allotropes, more than any other element in the periodic table. The most common state is the solid S8 ring, as this is the most thermodynamically stable form at room temperature. Sulfur exists in the gaseous form in five different forms (S, S2, S4, S6, and S8). For sulfur to convert between these compounds, sufficient heat must be supplied.However, the abundance of sulphur in the earth’s crust is only 0.03-0.1%. Combined Sulphur predominantly exists as sulphates and sulphides in metal ores. For example, sulphates such as Gypsum salt CaSO4.2H2O, Epsom salt MgSO4.7H2O, Baryte BaSO4andsulphides such as Galena PbS, Zincblende ZnS, Copper pyrites CuFeS2. Traces of sulphuralso occur in the volcanoes hydrogen sulphide. Sulphur is also present in organic materials such as eggs, proteins, garlic, onion, mustard, hair and wool.
Selenium naturally exists as a red or black amorphous solid, or a red or grey crystalline structure. The crystalline structure is the most stable.It is rare to find selenium in its elemental form in nature; it must typically be removed through a refining process, usually involving copper. It is often found in soils and in plant tissues that have bioaccumulated the element.A trace amount of selenium and tellurium are also found in sulphide ores as metal selenides andtellurides.
Tellurium is the metalloid of the oxygen family, with a silvery white colour and a metallic lustre similar to that of tin at room temperature. Tellurium is an extremely rare element and is most commonly found as a telluride of gold.
Polonium is a very rare, radioactive metal. About 33 different isotopes of the element can be synthesized and all of the isotopes are radioactive. It exists in a variety of states and has two metallic allotropes. It dissolves easily into dilute acids. Polonium and Livermorium do not exist in nature in compounds, but they can be formed synthetically in the laboratory. Polonium found in nature as a decayproduct of thorium and uranium minerals.