p-Block Elements: Group 17
Occurrence
At room temperature, halogens have first two elements in a gaseous state, next in liquid and last two solid matters in which astatine is a metalloid. In pure form, these elements are found as diatomic elements. However, the pure form themselves are highly reactive. Accordingly, halide salts are the best sources of halogens except for iodine. Halide ions can be oxidized to form free diatomic halogen molecules by various methods. This depends on the tendency of displacement and the ease of oxidation of the halide ion. Fluoride is the most difficult to oxidize as it has the highest electronegativity, whereas iodide is the easiest.
Fluorine is majorly manufactured using electrolytic oxidation of a fluoride salt. One such procedure is to use a molten potassium hydrogen fluoride, KHF2, and anhydrous molten hydrogen fluoride. Electrolysis causes HF to decompose, to release fluorine gas at the anode and hydrogen at the cathode. These two gases are needed to be separated to avoid their explosive recombination to reform hydrogen fluoride.
Most commercial chlorine comes from the electrolysis of the chloride ion in aqueous solutions of brine or NaCl. Chlorine is also a product of the electrolytic production of several metals such as potassium, calcium, and magnesium from their chloride salts. A strong oxidization agent may also be employed to prepare chlorine by the chemical oxidation of the chloride ion in acid solution. Strong oxidizing agents include manganese dioxide (MnO2) or sodium dichromate (Na2Cr2O7). For example:
MnO2(s)+2Cl−(aq)+4H3O+(aq)⟶Mn2+(aq)+Cl2(g)+6H2O(l)
Additionally, bromine is prepared by a displacement reaction between bromide and chlorine. The reaction is possible because chlorine is higher in reactivity than bromine. Likewise, bromide oxidizes to its liquid form while chlorine ionizes into a salt.
2Br−(aq)+Cl2(g)⟶Br2(l)+2Cl−(aq)
All domestic bromine is commercially manufactured using this procedure predominantly.
Likewise, in a similar trend, some of the iodine is obtained from the oxidation of iodine chloride, ICl, or iodic acid, HlO3. The commercial preparation utilizes the reduction of sodium iodate, NaIO3. This salt is extracted as an impurity in deposits of Chile saltpetre, with sodium hydrogen sulfite:
2IO−3(aq)+5HSO−3(aq)⟶3HSO−4(aq)+2SO2−4(aq)+H2O(l)+I2(s)
Since halogens are rarely found in their pure form, several halides occur in large quantities in the earth’s crust. The ocean and underground brine contain several types of halides. For example, magnesium chloride in the ocean is used to produce magnesium and its product is chlorine gas. Large underground deposits of NaCl have been located at several rocky salt mines around the world. These deposits provide sodium, chlorine with traces of other halides as well. The concentration of the chloride ion in brine is 0.54 M while other halides are less than 10–4 M.
Fluoride is found in mineral ores such as CaF2, Ca(PO4)3F, and Na3AlF6. Chloride and bromide can be located in the Great Salt Lake, the Dead Sea, sea kelp and other underground brine of Chile saltpetre. The extensive salt beds contain a mixture of NaCl, KCl, or MgCl2. In the human body, chlorine is present in the form of hydrochloric acid, which is a component of stomach acid as well as ions in the bloodstream.
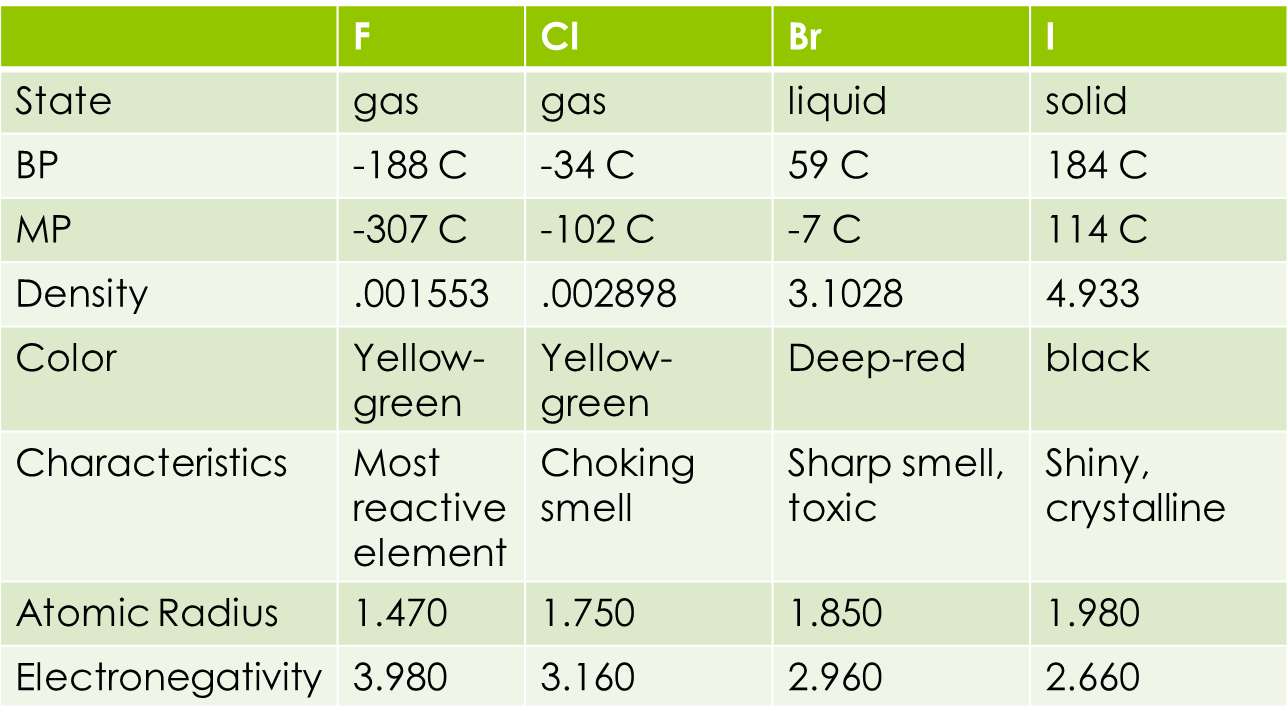
Fig 1: Some characteristic properties of halogens.