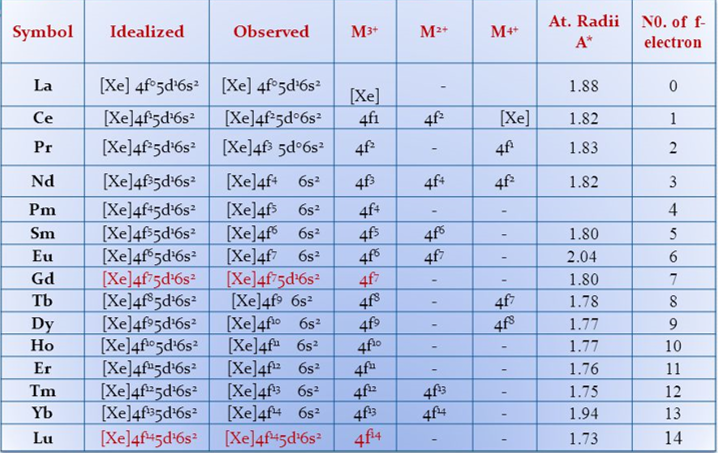
It has been shown that the lanthanide elements are highly electropositive and form essentially ionic compounds. It is observed for these elements that +3 (i.e. formation of tripositive ions, Ln3+) is the principal or common oxidation state exhibited by all of them. This is said to be the most stable oxidation state of the lanthanides. The magnitude of the energy required to remove an electron from the gaseous ion in its lower oxidation state (i.e. ionization energy) and of that released when two gaseous ions combine with water to form the aquated species (i.e. hydration energy) are such that all the tetrapositive species (except Ce+4) and all the dipositive species (except Eu+2) revert to the tripositive species. Thus this leads to the conclusion that tripositive species are more stable than the di- and tetrapositive species in aqueous solution.
Some of these elements also show +2 and +4 oxidation states but except a few such ions, they have the tendency to get converted to +3 state. For example, Sm and Ce form Sm2+ and Ce4+ ions but are easily converted to +3 states. That is why Sm2+ is a good reducing agent while Ce4+ is a good oxidising agent, i.e.,Sm2+ → Sm3+ + e (electron donor and Ce4+ + e → Ce3+ (electron acceptor). Ln2+ and Ln4+ ions are less frequent than Ln3+ ions among the lanthanides.
The +2 and +4 oxidation states are shown by the elements particularly when they lead to a Noble gas electronic configuration, e.g., Ce4+ (4f0 ), Half-filled f-orbital, e.g., Eu2+ and Tb4+ (4f7 ), and a completely filled f-orbital, e.g., Yb2+ (4f14) in the valence shell. Among the above, +2 and +4 oxidation states, which exist only in aqueous solutions, are exemplified by Sm2+, Eu2+, Yb2+ and Ce4+. There are some exceptions also, i.e., sometimes +2 and +4 oxidation states are also shown by the elements which are close to f0, f7 and f14 states, e.g., the valence shell configurations of the ions given below are 4f1, 4f2, 4f3, 4f6 and 4f8, etc.:
Ce3+: 4f1 ;
Ce2+: 4f2 ;
Sm2+: 4f6 ;
Pr4+: 4f1 ;
Pr3+: 4f2 ;
Dy2 : 4f8 ;
Nd4+: 4f2 ;
Tm2+: 4f13
No satisfactory explanation for these exceptions has yet been given. These oxidation states have only been explained on the basis of thermodynamic and kinetic factors, that too arbitrarily. Due to the only one stable oxidation state (i.e., +3), lanthanide elements resemble each other much more than do the transition (or d block) elements. It has also been observed that the higher oxidation states of the lanthanides are stabilized by fluoride or oxide ions, while the lower oxidation states are favoured by bromide or iodide ions. Among the lanthanides, in addition to +3 states, +2 states are shown by Nd, Sm, Eu, Tm, and Yb only whereas +4 state is exhibited by Ce, Pr, Nd, Tb and Dy elements. Rest five elements show only +3 states.