The relative tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called electronegativity.
The electronegativity of an atom depends on the following factors:
- Size of the atom: In a smaller atomic shell is placed close to the nucleus. So the nucleus is able to attract bonding electrons effectively.
- Nuclear charge: Large nuclear charge can attract bonding electrons effectively.
- Shielding effect: (shielding effect s > p > d > f). Strong shielding defends outer electrons from the nuclear attraction while weak shielding enables the nucleus to attract outer electrons as well as bonding electrons more powerfully.
- Electronic configuration: Elements with near half filled or full filled configuration have a strong desire to get possession of bonding electrons so that they can achieve a stable configuration.
The electronegativity increases from left to right in a period and decreases from top to bottom in a group. The attraction between the outer most electrons and the nucleus decreases as the atomic radius increases which in turn decreases the electronegativity. The trend of electronegativity is similar to that of ionization enthalpy. Likewise, non-metallic elements have a stronger tendency to gain electrons in comparison to metallic elements. Atoms with low ionization energies have low electronegativities because their nuclei do not have a strong attraction for electrons. Those Atoms that are high ionization energies and they have high electro negativities because the nucleus has a strong attraction for electrons. So, the atoms of nonmetals group 7A (halogens) are the greatest in the electronegativity, while the atoms of the alkali metals group 1A are lowest in the electronegativity.
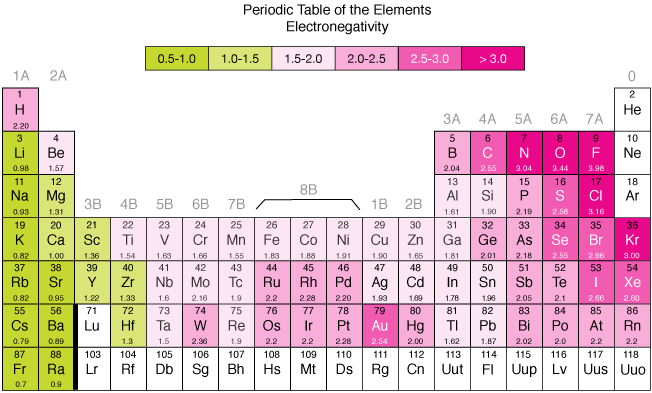
Figure 1: Periodic trends – The electronegativity increases from left to right in a period and decreases from top to bottom in a group.
In the periodic table, the hydrogen atom has a bare proton relatively unshielded by its single 1s electron. It has a middle-of-the-road electronegativity, reflecting its high propensity for forming covalent bonds. According to the Pauling scale s, the electro negativities of nitrogen and oxygen are respectively 3.0 and 3.5. In both cases, the bonding electrons are in the 2-level and screened from the nucleus by the 1s electrons. But oxygen has 8 protons in the nucleus whereas nitrogen only has 7. A bonding unit of 2 things will go through more attraction from the oxygen’s nucleus than from nitrogen’s, and so the electronegativity of oxygen is greater. Similarly, when Sulphur and oxygen are compared, Sulphur’s bonding electrons are in the 3-level and are shielded from the 16 protons in the nucleus by a total of 10 electrons in the 1- and 2-levels. The outer electrons, thus, goes through a net pull from the nucleus of 6+. With oxygen, the bonding electrons are at the 2-level, and the 8 protons in the nucleus are shielded by the 2 electrons in the 1s orbital. Again, there is a net pull of 6+ from the nucleus. However, the bonding electrons in the sulphur are further from the nucleus, and so the attraction is lessened. So sulphur is less electronegative than oxygen. Fluorine with the value of 3.96 is the most electronegative element, whereas francium and caesium with the value of 0.7 is the least electronegative element.