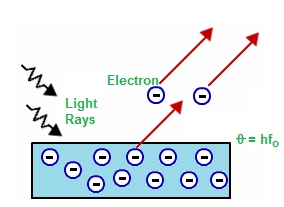
When light hits any material there is an emission of electrons and other free carriers and this whole process is called photoelectric effect and in the manner that electrons were emitted is called photoelectrons. According to many theories, this effect can be concluded from the transfer of energy from light to an electron so from this perspective an alteration in the intensity of light could induce some kind of changes in kinetic energy of the electrons emitted by the metals. To understand it clearly the perception of photons of a light beam can be taken as its characteristic energy which is proportional to the frequency of light. In a process called the photoemission process, an electron within a material that absorbs the energy of one photon and can acquire more energy than the work function of the material on the other hand if the energy of the material is low the electrons are somehow unable to escape the material.
What are the uses of photoelectrons?
Photoelectric has an abundance of uses as a photomultiplier, image sensor, gold leaf electroscope, photoelectron spectroscopy, spacecraft, moon dust, night visions, and many more useful things. Most commonly if an example is taken it is of moon dust as we all know light from the sun which hits the lunar dust is the main cause of making it charge of the photoelectric effect and these charges later affect the moon and came to be known as electrostatic levitation. Night vision is a very prominent example of photoelectric effect as it can make visible or shoot anything at a very low light possible by it’s the effect of electrons which are present and work with the metals involved. Another example of this is spacecraft as the photoelectric effect will cause the spacecraft exposed to the sunlight to develop a positive charge.
What is the mechanism of the photoelectric effect?

It is said that not every electromagnetic wave can cause the photoelectric effect but only radiation of a certain frequency will cause the effect. Work function will be found by a cutoff frequency which is somehow the amount of energy that holds metals to the metal surface whereas work function is a property of the metal which can not be affected by incoming radiation. Only when a frequency of light that strikes the metal surface which is greater than the cutoff frequency then only the emitted election has some kinetic energy otherwise it is not possible to find any.