p-Block Elements: Group 17
Trends in physical and chemical properties
All Group 17 elements are non-metals. Hence, they are insulators of heat and electricity. The density of halogens increases down the group. Halogens have low melting and boiling points which increase down the group. The forces of attraction between the molecules go weaker as the atomic size increases down the group. As the size increases, the van der Waal`s forces of attraction between the molecules become stronger. More heat is required to overcome the attractive forces and therefore the melting points and boiling points increase. The first two elements (fluorine and chlorine) are gases at room temperature. Bromine is a liquid whereas iodine and astatine are solids at room temperature. The halogen obtains a darker colour down the group.
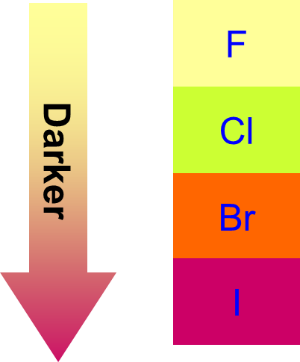
All halogens have high electro-negativities. They are electronegative non-metals. The electronegativity of halogens decreases down the group from chlorine to iodine. As the atomic radius becomes larger down the group, the forces of attraction between the nucleus and electrons become weaker and thus electronegativity decreases. The attraction of halogens for an extra electron increases up to the group: I<Br<Cl<F. Therefore, electron gain affinity increases while the first ionization energy decreases as the atomic size increases.
The bond dissociation energy decreases from Cl to me while the bond length increases. Fluorine has a small atomic size and compact volume. There is a strong repulsion among non bonded electrons. The bond dissociation energy of fluorine is lesser than chlorine and bromine.
The atomic radius of halogens increases down the group as the number of electrons increase. Thus, the forces of attraction between the nucleus and the electrons become weaker. As a result, the halogen lower in the group has a lower tendency to attract an electron to form a negative ion. Ions are larger than atoms which means that the added electron repels the others, so radius gets larger.
All Group 17 elements are very reactive, and they have 7 valence electrons. The atoms get bigger as you go down the group and that decreases the reactivity. This reduces the attraction of the (+) nucleus for an additional (-) electron. The chemical reactions of Group 17 elements involve the formation of negative ions of charge –1: Cl–, Br–, I–etc. Chlorine, bromine and iodine have similar chemical properties, but they are different in terms of their reactivity. They dissolve in water to obtain strong acidic solutions. Reaction with NaOH produces sodium halides, sodium halate(I) and water while reaction with Fe produces iron (III) halides.
| Reaction with hydrogen | Reaction with sodium hydroxide | |
| F2 | Reacts instantly even at -200°C | Colourless fluorine gas dissolves in a rapid reaction to form a colourless solution |
| Cl2 | Reacts slowly in dark. Explodes in light | greenish chlorine gas dissolves to form a colourless solution. |
| Br2 | Needs heating to +200°C in order to react | brownish bromine water dissolves steadily to form a colourless solution |
| I2 | Does not react completely even at 500°C | dark iodine crystal dissolves slowly to form a colourless solution. |
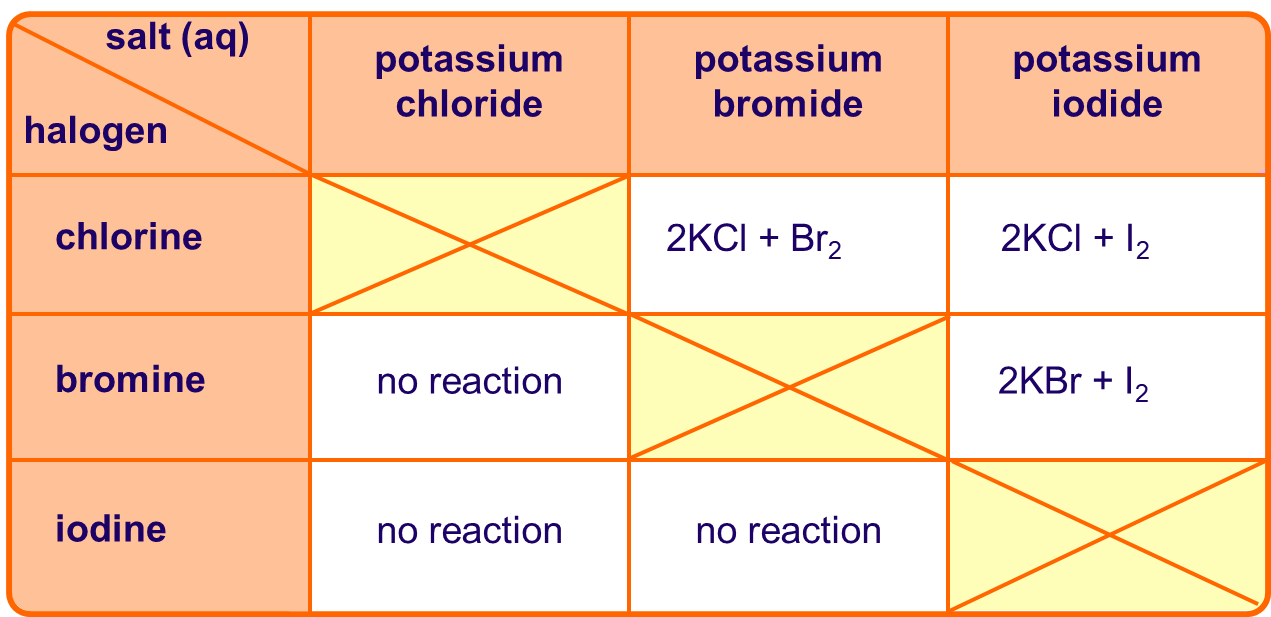
Displacement reactions:
Chlorine is capable of oxidising both bromide and iodide ions:
Cl2 + 2Br– 2Cl– + Br2
Cl2 + 2I– 2Cl– + I2
However, bromine can only oxidise iodide ions:
Br2 + 2Cl– no reaction
Br2 + 2I– 2Br– + I2
So, chlorine is a stronger oxidising agent than bromine.
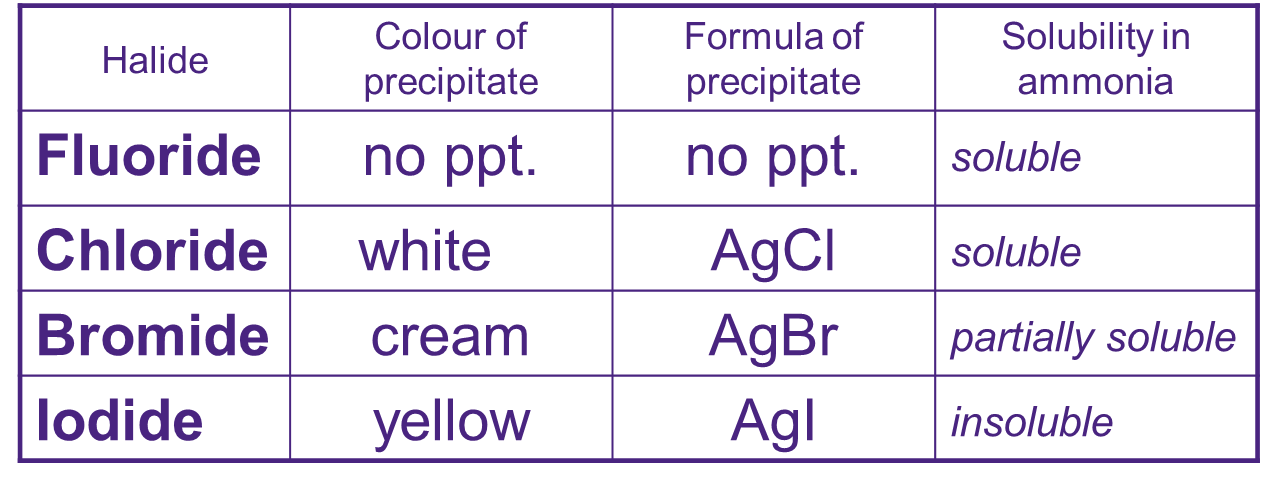
Reactions with Silver Nitrate produces silver precipitates. Halides precipitate as follows
Ag+(aq) + X¯(aq) Ag+X¯(s)
when they dissolve in ammonia a colourless diamine complex is formed [Ag(NH3)2]+(aq)
Titration of Iodine with Sodium thiosulphate, Na2S2O3
I2(aq) + 2Na2S2O3(aq) 2NaI(aq) + Na2S4O6(aq)
or(ionic equation):
I2(aq) + 2S2O32-(aq) 2I– (aq) + S4O62-(aq)
The colour change observed at the endpoint of this titration is blue-black colourless.