p-Block Elements: Group 18
Physical and chemical properties
All noble gases are colourless, odourless and unreactive. This makes it difficult to isolate and identify. Nowadays, a number of compounds of these gases, particularly of xenon and krypton have been prepared, this shows that these gases are not completely inert. They are also called rare gases because of the low abundance of these gases on earth. Due to no or minimal reactivity of noble gases, there are few patterns, or trends, among the group. All Group 8A elements are monatomic gases at standard temperature and pressure. But they can be solidified at extremely low temperatures, due to weak (induced) van der Waals forces between the atoms, e.g. neon freezes at -249°C, helium at -270°C. The larger the atomic mass, the lower the freezing temperature (as the van der Waal’s forces are stronger between larger atoms with more electrons to contribute to the inter-atomic attractive forces). The solids have the face-centred cubic (fcc) close-packed arrangement.
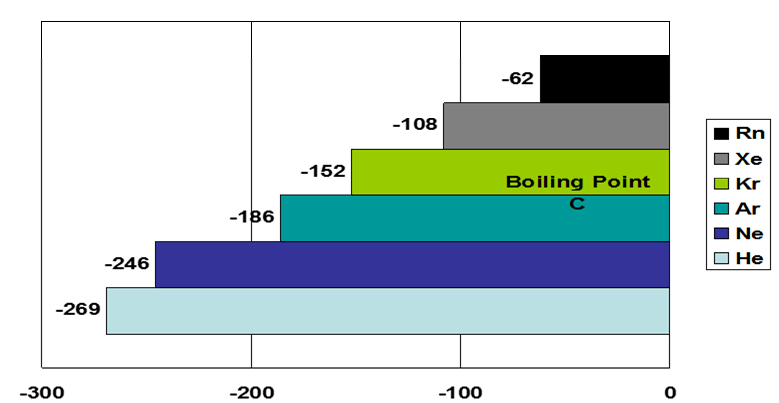
For example, the density of the noble gases increases down the group as the atomic size increases. The boiling point also increases down the group.
Ionisation Enthalpy is very high ionisation enthalpy due to stable electronic configuration, it decreases down the group with an increase in atomic size. Atomic radii increase down the group with an increase in atomic number. Due to stable electronic configurations, noble gases show no tendency to accept the electron. Therefore these gases have large positive values of electron gain enthalpy which decreases down the group.
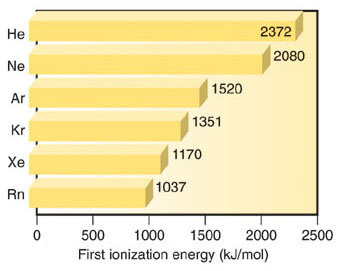
| Element | Outer
electronic configuration |
Vander
Wall’s radius (Å) |
First
IE (kj mol-1) |
m.pt.
(K) |
b.pt.
(K) |
∆Hfux
(kj mol-1) |
∆H vap
(kj mol-1) |
| He
Ne Ar Kr Xe Rn |
1s2
2s2 2p6 3s2 3p6 4s2 4p6 5s2 5p6 6s2 6p6 |
–
1.31 1.74 1.89 2.10 2.15 |
2372
2080 1519 1351 1170 1037 |
–
24.4 83.6 116 161 200 |
4.2
27.1 87.3 120 166 211 |
0.02
0.33 1.18 1.64 2.3 2.9 |
0.08
1.77 6.5 9.0 12.6 16.4 |
Chemical Inertness of noble gases is supported by the following reasons:
- The atoms have stable completely field electronic shells with a complete octet
- They have high ionisation energies and negligible electronegativity
- They have almost zero electron affinities.
Therefore, they do not have any tendency to gain, lose or share electrons with other atoms. Under excited condition:- Sparking Helium at low pressure in the presence of mercury, tungsten etc. forms compounds like HgHe2, HgHe10, WHe2. Helium compounds are also formed in discharge tubes like BiHe2, FeHe, Pt3He, PdHe. These compounds are not considered as true chemical compounds because He is absorbed on the surface and they are essentially unstable. Compounds formed through co-ordination- Argon forms a number of the unstable compound with varying no. of BF3 molecules e.g. Ar.BF3, Ar.6BF3.In these compounds, argon atoms donate a pair of electrons to Boron atom of BF3. In case of higher compounds fluorine atoms of BF3 also donate a pair of electrons.
The hydrates of noble gases are produced by compressing the gases with water e.g., Xe.6 H2O. Compounds then take shape and evolve by physical trapping (Clathrates). The inert gases Argon, Krypton and Xenon can be found as solid compounds. Certain organic molecules such as phenol and hydroquinone may contain noble gases when combined under pressure. In such compounds, the inert gas is enclosed in the crystal lattice of organic compounds known as clathrates or cage compounds.