Polar Covalent Bond
A covalent bond between the different atoms has the possibility of acquiring the ionic characters that can be described on the basis of electronegativity concept. Electronegativity of the element is illustrated as the power of its atoms for attracting “d” pair of electrons or bonding towards itself.
Polar Character of covalent bond
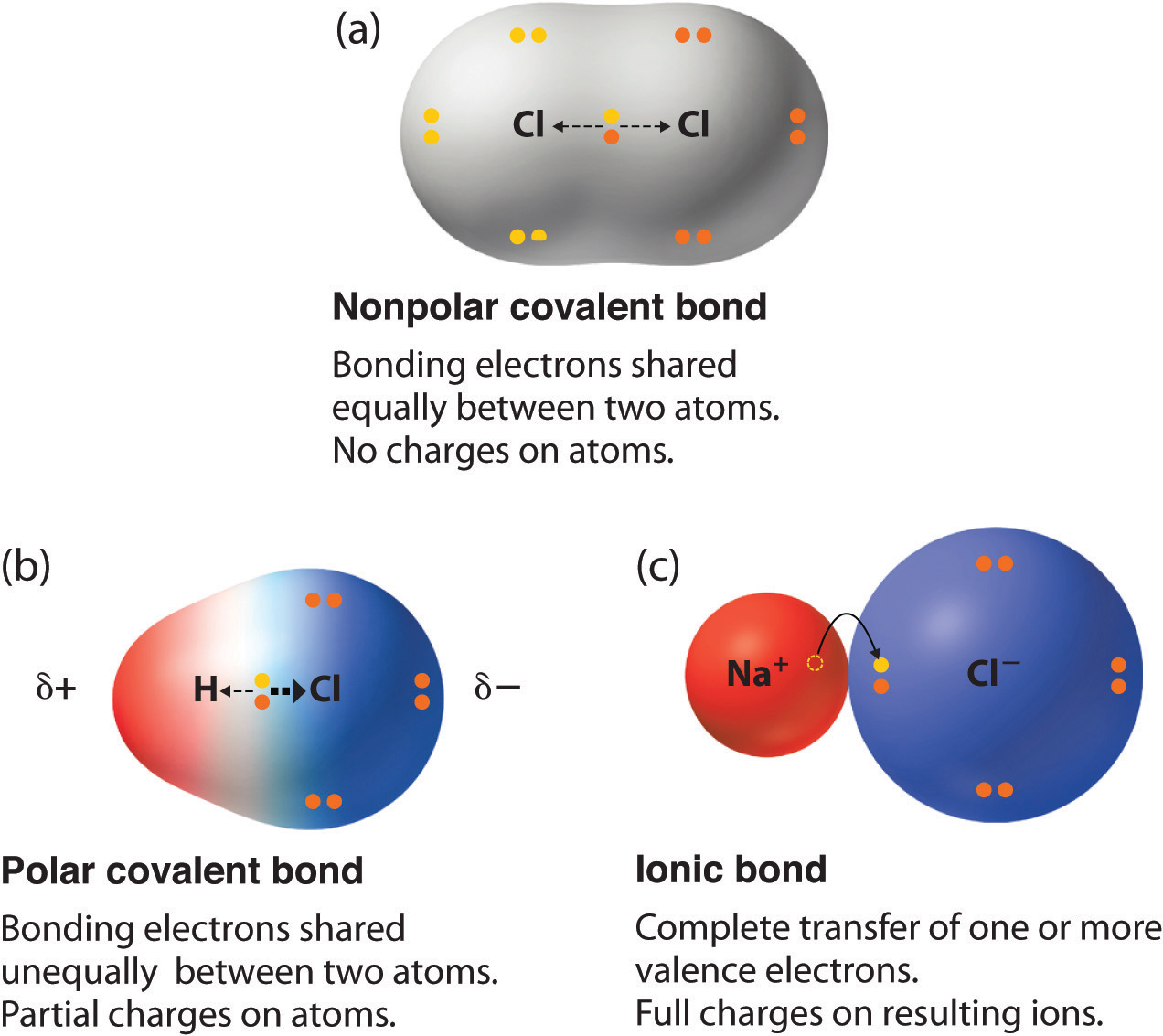
When the covalent bond is formed between two identical atoms, then the “shared pair of electrons” are located halfway between the nuclei of 2 atoms, as both atoms gain the same attraction for “bonding electrons”. The atomic orbital or electron cloud that creates covalent bonds is distributed symmetrically around atoms. This type of covalent bonding is known as a non-polar covalent bond.
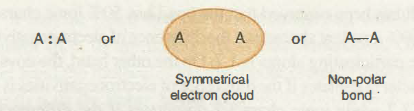
For instance, molecules such as H2, N2, O2 and Cl2, all consist of non-polar bonds.
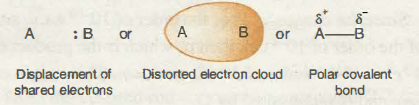
If in any case, there’s the formation of a covalent bond between two dissimilar or unidentical atoms, one out of which bears a large electronegativity value, the bonding electrons pair is distributed towards higher electronegative atom. Specifically, the electron cloud which contains the bonding electrons receive distortion and the charge density is more concentrated around more atom which is having higher electronegativity.
Due to the uneven distribution of electron charging density, more electronegative atom receives limited negative charge (pointed out as 8-, in fact, less electronegative atom acquiring a limited positive charge being indicated as 8+). Therefore, a covalent bond advances to the partial ionic character as a consequence of the dissimilarity of the atoms’ electronegativities which is comprised of the bond. This type of bond is known as a polar covalent bond, shown in the figure below.
For instance, the bond present between H and Cl atoms in the HCl molecule is of polar nature as the shared pair of the electron is dislocated towards the Cl (chlorine) atom that is having higher electronegativity.
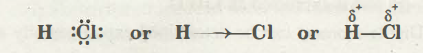
For example, hydrogen fluoride is more polar than hydrogen chloride because the difference between H and F is more than that between H and Cl
The limit of an ionic character in the covalent bond is dependent on the electronegativities’ difference of two atoms which form a bond. The greater the electronegativity difference, the higher is the ionic character percentage in a bond. Let’s say, Hydrogen Flouride (HF) has higher polarity than Hydrogen Chloride due to H and F differences which are higher than between Hydrogen (H) and Chloride (Cl).
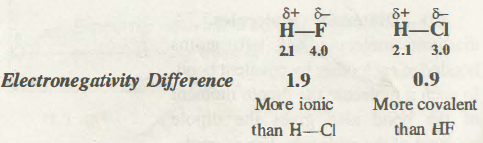
There has been this notice that the bond features half ionic character and half covalent character, if electronegativity difference is 1.7 of the participating atoms, on the flip side, the covalent character becomes dominant if the electronegativity difference is lower than 1.7, and the ionic character leads if the electronegativity difference is more than 1.7.
Summary
Compounds containing polar covalent bonds consist of electrons which are unevenly shared between bonded atoms. The polarity of this type of bond is decided mostly by respective electronegativities of bonded atoms.
The asymmetrical distribution of charge in polar substances produce dipole moments, which refers to the product of incomplete/partial charge on bonded atoms and the defined distance between them.