Diazonium salts are included in the group of organic compounds and they are also known as the diazonium compounds. If they are heated in the presence of water, they make the phenols. benzene diazonium hydrogen sulfate and benzene diazoniumchloride are the examples of diazonium salts. The process of making the diazonium salts is quite simple and is known as the diazoniation or diazotization. The reaction of aryl or alkyl primary amine with sodium nitrite in the presence of hydrochloric acid produces the diazonium salts. The reaction conditions are mild for most of the time and this reaction can occur at the room temperature or even below that temperature.
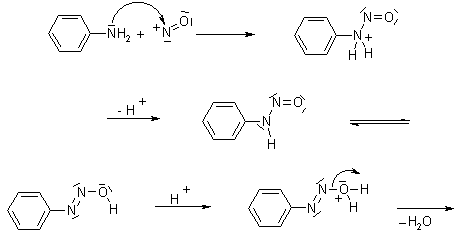
The reaction between the phenylamine and nitrous acid at the temperature less than the 5 degree Celsius causes the production of diazonium salts. The reaction between the nitrous acid and aniline results in the formation of benzene diazonium chloride. As nitrogen is highly toxic gas so this reaction is conducted in the in-situ conditions, by reacting with a mineral acid NaNO2. It is necessary to keep the temperature less than 5 degrees Celsius during the reaction conditions as the temperature above 5 degrees Celsius causes the diazonium salts to be unstable. In this reaction, first, the solution of phenylamine is dissolved in the hydrochloric acid followed by adding the potassium, or sodium nitrile solution. In this reaction nitrous acid is produced and depending upon the temperature, the phenylamine reacts with the nitrous acid. If this reaction happens during the warm conditions, a black oily product is obtained that contains the phenol and the nitrogen gas. If the same reaction occurs in a beaker of ice, at the low temperatures, then the solution of potassium or sodium nitrite is cooled in the ice. Then to the phenyl ammonium chloride solution, nitrite solution is added very slowly and resultantly a solution containing the benzene diazonium chloride is obtained. In this reaction, the positive ion that contains the N+ group is known as the diazonium ion. A bit of its name the azo refers to the nitrogen.
Azo coupling is the most widely used reaction for the preparation of diazonium salts. In this reaction, the compound of diazonium is attacked by the electron-rich substrates. The resulting compounds are often used for the dyes. Under normal conditions, the diazonium ion is highly unstable, so, generally, it is not stored and is immediately used after its preparation.