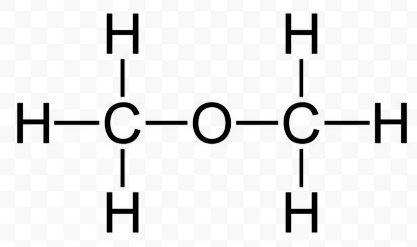
The ethers are compound with alkoxy groups. The ether groups are of R-O-R’ type, where the R and R’ are alkyl group with an oxygen atom in the middle. The alkyl groups could be the same or different. They nomenclature of others doesn’t follow some rule as such. In accordance with different alkyl groups attached to the ethers, different suffixes are given to the ethers. The electron density of ethers is quite high due to the alkyl groups attached to the oxygen. This makes the ether more sort of electron donating groups.
On a major basis the ethers are classified into the symmetrical and asymmetrical ether. There are different types of ethers R-O-R, R-O-A , A-O-A, where the R is alkyl group and A is the aryl group.
Different preparations of phenols:
Preparation by dehydration of alcohols
One of the methods of preparation of ethers is by the dehydration of alcohols. The dehydration process is done in the presence of protic solvents. The proticsolvent like sulphuric acid is used in the preparation process. Along with the ether produced we may also get alkenes by the dehydration observed at different temperature.
The preparation of ethoxyethane can be observed by the dehydration of ethanol along with sulphuric acid at 413K. This is one of the major processes used by the makers for preparation of ethers by the usage of primary alcohols.
This reaction proceeds by the nucleophilic substitution reaction. In this process, the alcohol serves for two purposes. The alcohol serves as a nucleophile in one place and as a substrate at another place. Hence the same alcohol serves for two roles in the dehydration process.
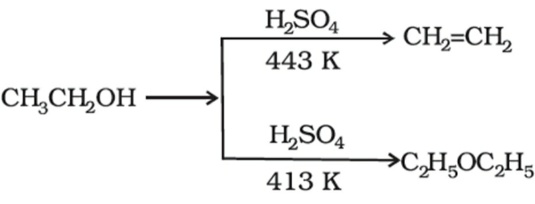
The process of reaction may be of SN2 or SN1. The reaction will take SN1 or SN2 path would be decided by the degree of carbon with which the alcohol is attached. Generally, the primary alcohols follow the SN2 mechanism of nucleophilic substitution. Whereas the secondary and tertiary alcohols follow the SN1 mechanism of nucleophilic substitution.
Preparation of ether by Williamson’s reaction:
This is an important process for the preparation of symmetrical and asymmetrical ethers. It is a worldwide used process for the preparation of ethers. In this reaction, the alkyl halide is reacted with sodium alkoxide. The reaction at the end gives ether as the final product. The type of mechanism followed by the reaction is SN2 mechanism for the primary alcohols. This is because of the type of reaction observed is the elimination reaction. As we know the alkoxides are strong bases and react readily with alkoxide. The yield of the reaction is higher for primary alcohols than secondary and tertiary alcohols.