In the world of Quantum Systems quantization means the storing of energy in terms of quantas (i.e packets of energy).Quantum numbers are basically numerical entities that depicts about the stored conserved energy of electrons in the atoms. Along with the numerical values the energy levels are also depicted by the Angular moment, spin of electrons, etc.
Types of Quantum numbers
In order to describe the electron in an atom, four types of Quantum numbers are used:
- Principal quantum number (n)
- Azimuthal quantum number (l)
- Magnetic quantum number (m)
- Spin quantum number (s)
Principal Quantum number (n)
This quantum number is assigned on the numbers of shells available to the atom or the energy levels of the electron. The values of ‘n‘ ranges from 1 to the number of shell containing the outermost electron of the atom.
For example, in Helium (He), the outermost valence electron is in shell of energy level 1, hence n = 1.
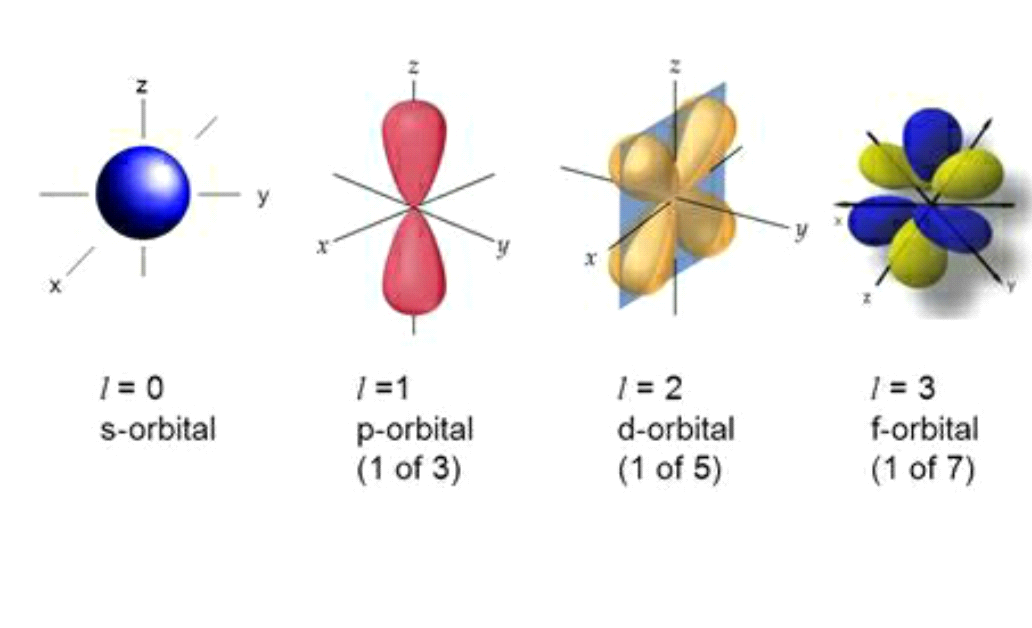
Azimuthal Quantum number (l)
Azimuthal quantum number or Orbital quantum number describes the subshell of the electrons. It also helps in the valuation of the angular momentum for electrons. For the field of Chemistry, l=0 is s orbital, l = 1 is p orbital, l=2 is d orbital and l=3 is f orbital. The value of l varies from 0 to n-1.
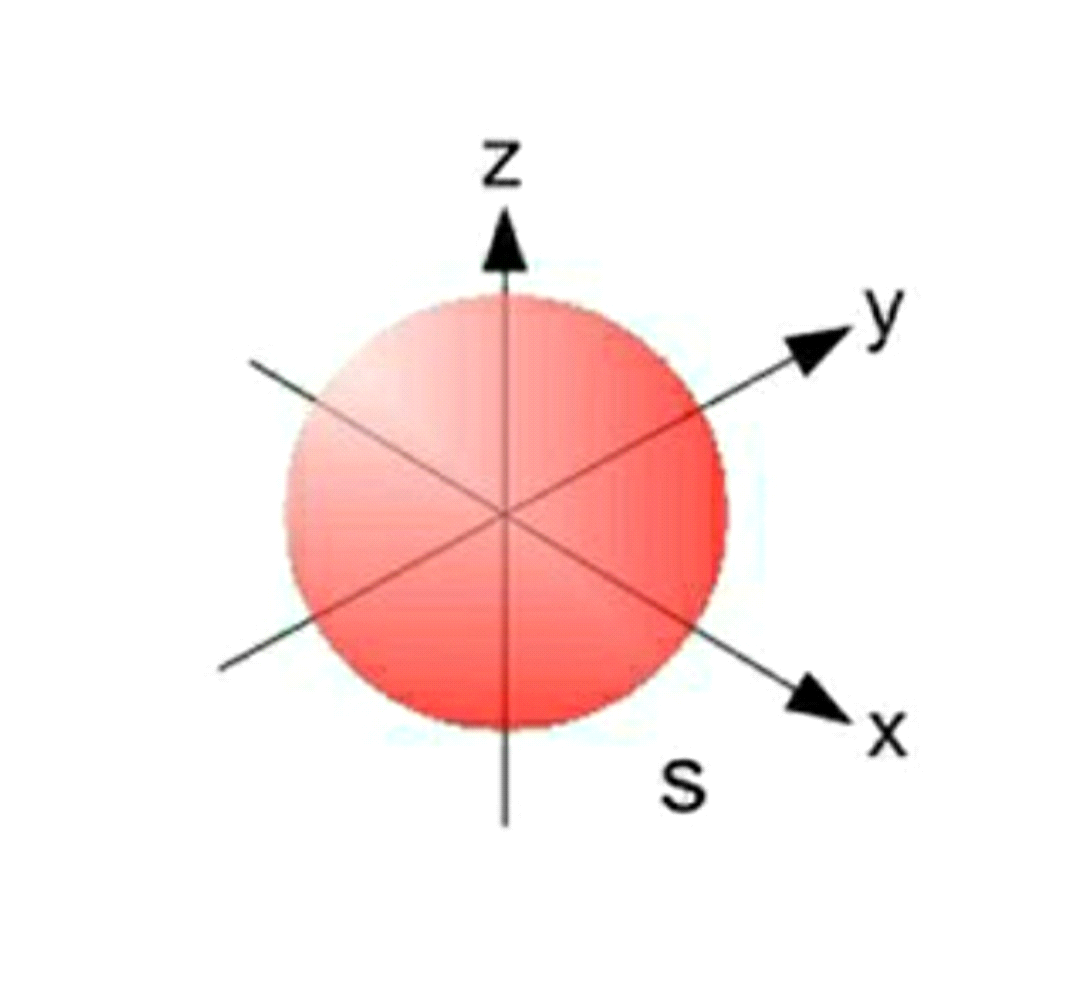
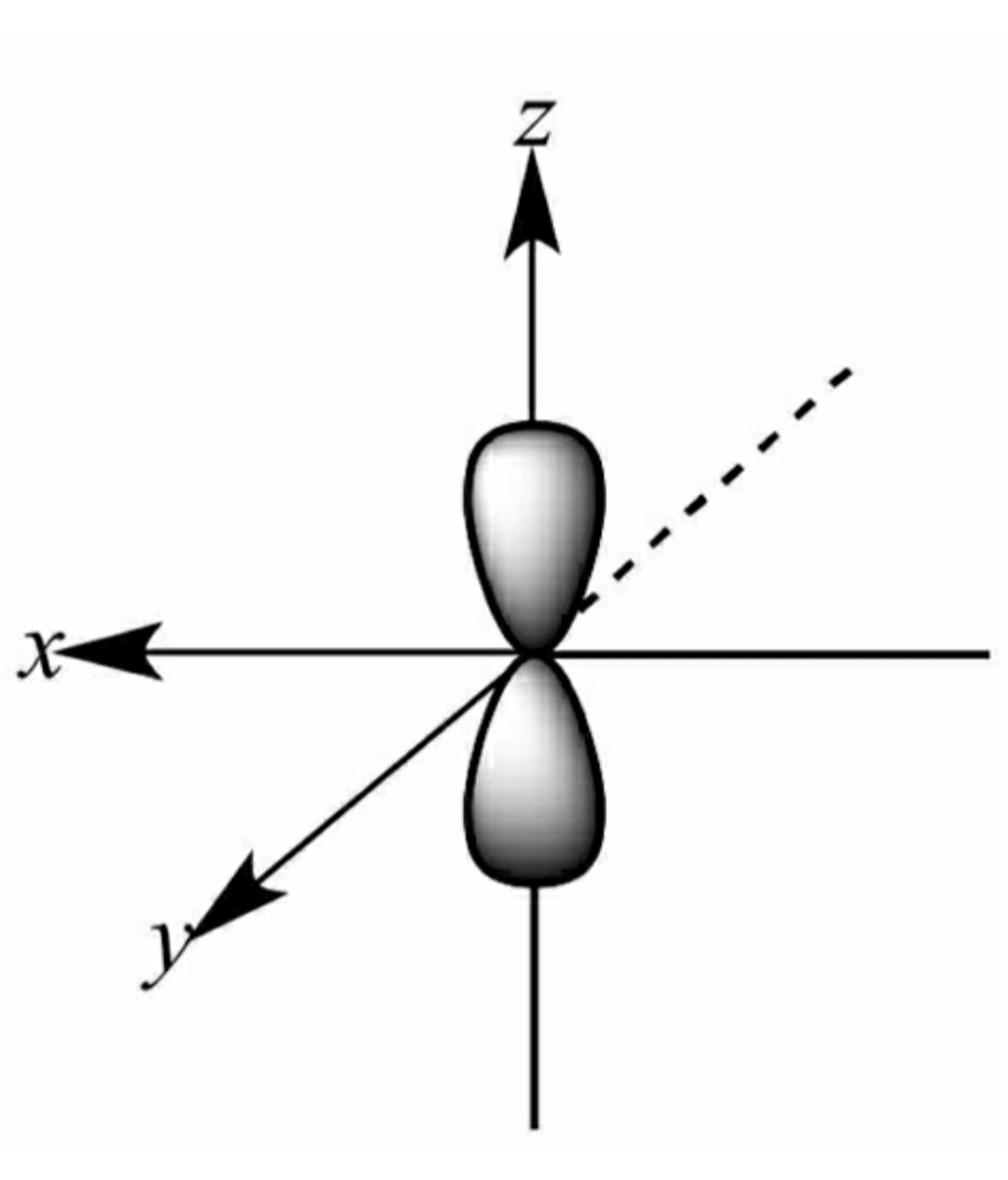
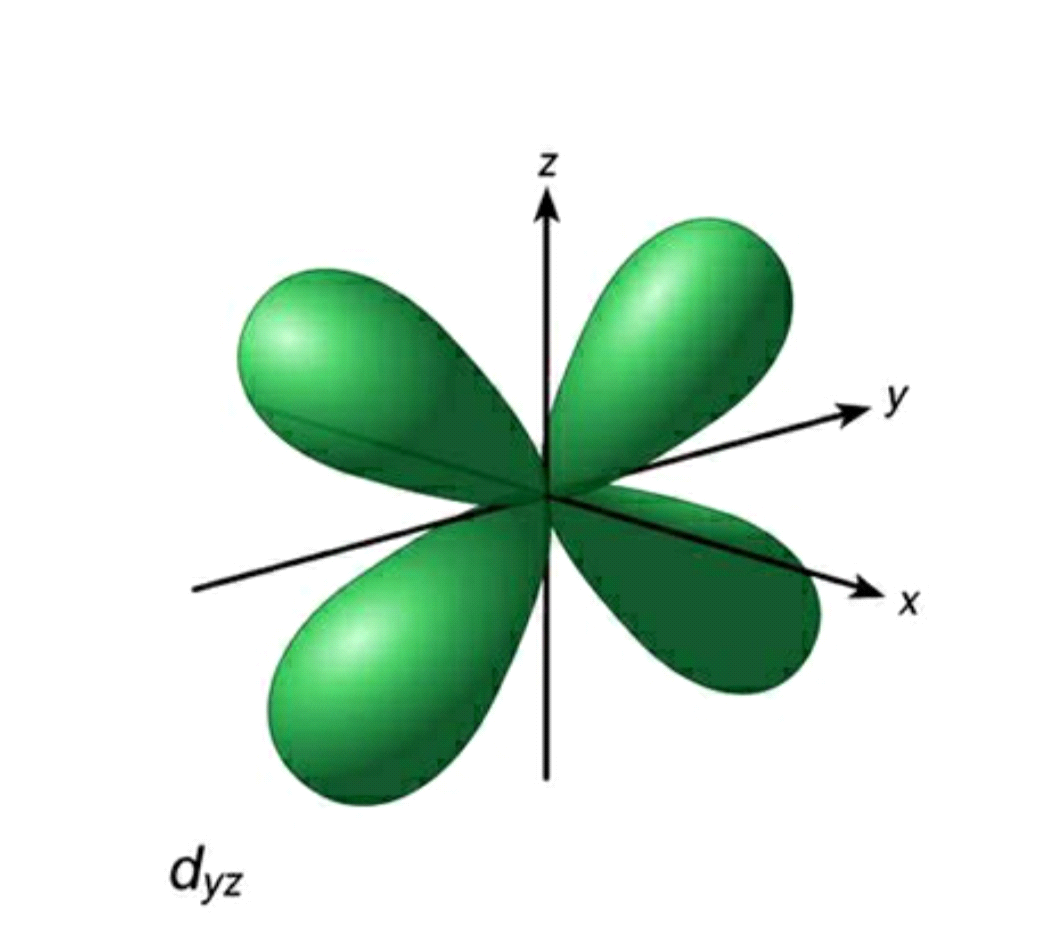
For example, for n=2, l = 0,1. Therefore the possible sets will be 2s 2p, where 2 is the energy level of electron and s and p are subshells.
Magnetic Quantum number (m)
The magnetic quantum number is for the description of the shape of the orbitals of the subshells. This is because it is the number of electron clouds (high density regions of electrons) there in the subshells orbitals. Also it helps in the knowing the nodes (0 electron regions) located in the orbitals.
The values of Magnetic quantum number varies from –l to +l. Therefore for s subshell, l= 0 so it has only one orbital. For p subshell, l = 1, hence it has 3 orbitals (m = -1, 0, 1). Similarly for d subshell there are 4 orbitals and so on.
Spin Quantum number (s)
It is an intrinsic property of the electrons in the orbitals. It depicts about the angular moment of the electrons along an axis.
An orbital contains a maximum of of 2 electrons in it with two different spins, hence each electron ha two different s values. It is not compulsory to take a specific value but in order to avoid any confusion we take 1/2 and -1/2 as the spin values. As both the electrons have the same energy level the numbers are same but dues to opposite spins different signs are allotted referring to up spin and down spin respectively.
Example:
Neon (Ne) has 10 electrons hence the configuration is 1s2 2s2 2p6.
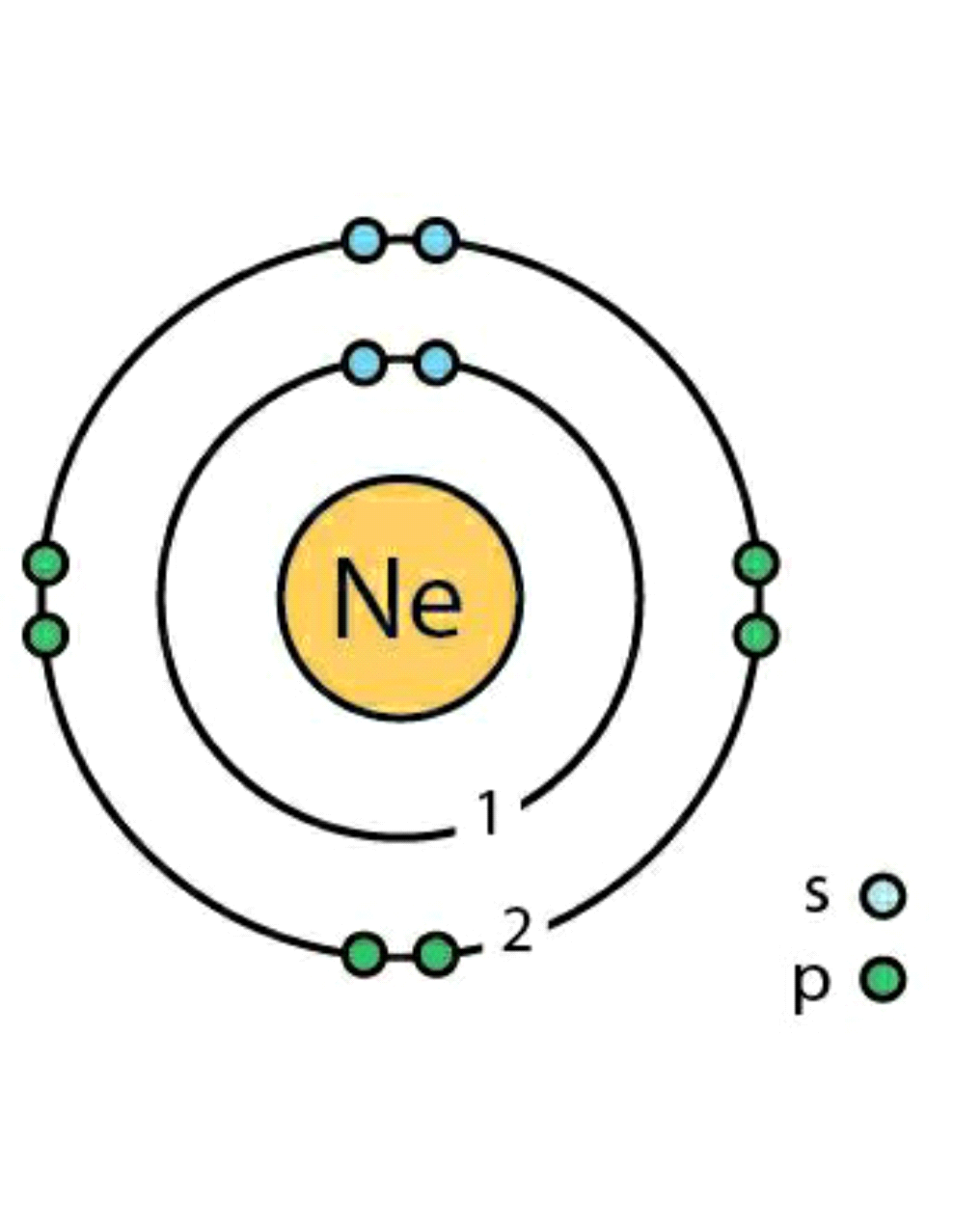
Now here we can see that, in He outermost shell is 2 hence n = 2. Therefore l = 0,1 as l ranges from 0 to n-1, so we have s and p orbitals for l values 0 and 1 respectively. Value of m will be 1 for s orbital, and 3 for p orbital respectively. In each orbital there will be two electrons with upward and downward spin (+1/2 and -1/2).
NOTE : There are many other factors and rules for the valuation of the energy levels to the electrons. It is not alone possible for the Quantum numbers to considerably give complete knowledge about the energy of the electrons in an atom.