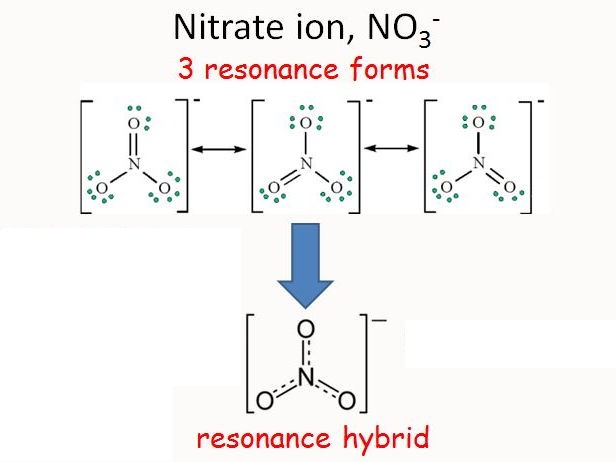
Resonance
Resonance dictates the method that is within the VB theory or Valence Bond Theory of bonding and the way of detailing the “delocalized electrons” within some molecules. Here, the bonding is not particularly described out by a lone Lewis structure. It definitely contrasts and compares two or more than two potential Lewis structures which are depicted by a specific molecule.
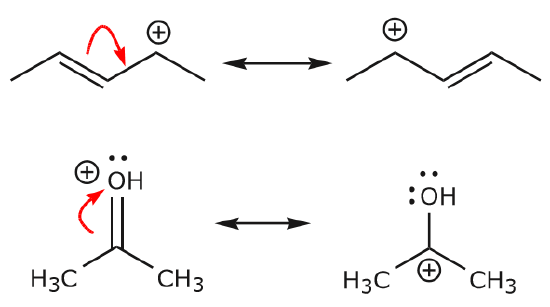
Each and every exclusive Lewis structure is known as a contributing structure (or arrangements, also diversely called resonance structure or canonical structure) of target ion or molecule.
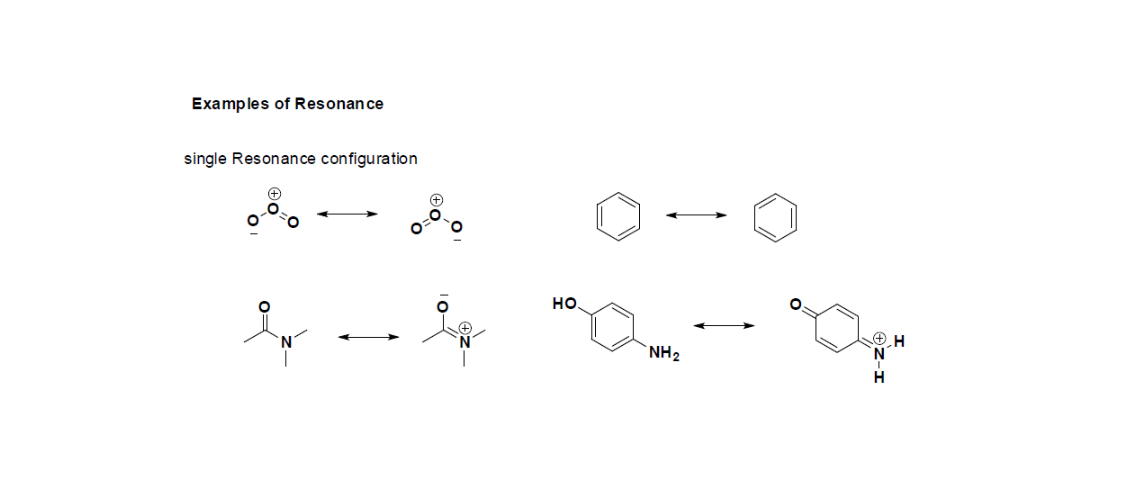
The Contributing structures are exactly not the isomers (an isomer is a molecule or ion with similar formula but different structure) of a target ion or molecule since they are only different considering the location of delocalized electrons.
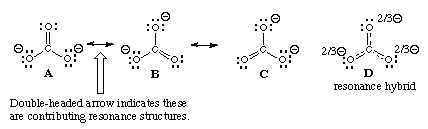
The above is an example of resonance
Resonance structures or contributing structures are in use when a single Lewis structure considering only one molecule can’t completely illustrate the bonding which occurs in between the nearby atoms comparative to the “Empirical data” for the definite bond lengths present between the particular atoms.
The final sum of “valid resonance structures” is described as the resonance hybrid. This resonance hybrid represents the complete electron delocalization inside a molecule.
A molecule containing many resonance structures is much more stable as compared to fewer resonance structures. A few of the resonance structures are more positive, favorable, or additionally supporting than others.
In atoms, molecules, and compounds, electrons don’t have fixed position but there’re probabilities to be found in some places (orbitals). The resonance forms describe the higher probabilities areas (electron densities). It’s just like keeping your hat either in your left hand or your right hand. When there are two or more than two possibilities available, then the word resonance has its role to play.
Resonance structures don’t alter atoms’ relative positions. Lewis structure’s skeleton remains exactly the same, electron locations are only changed.
Resonance Structures and Delocalization Rules
Electrons have the capability to move for helping to stabilize a molecule in resonance structures. This movement of electrons is referred to as delocalization.
- Resonance structures must consist of an identical number of (e−) electrons, no electrons should be added or subtracted. (Only check out the number of electrons by counting them).
- All resonance structures should be following the rules of drawing/writing the Lewis Structures.
- The hybridization of a structure should remain the same.
- The skeleton of a structure can’t differ to change (electrons can move only).
- In resonance structures, the number of lone pairs should be the same.
Formal Charge
Although the structures look alike, the (FC) formal charge might not be. Formal charge refers to the charge that is allocated to a particular atom in a molecule. If calculated rightly, the molecule’s net formal charge should be identical to a molecule’s oxidation charge (the calculated charge while writing out the molecular and empirical formula).
We wish to select the resonance structure with the smallest formal charges, which adds to zero or the overall molecules’ charge. The formula to find Formal Charge is-
Formal Charge = (no. of valence electrons in free orbital) – (no. of lone pair electrons) – ½ (no. of bond pair electrons)
The FC has to be equal to the overall charge of the molecule, e.g. the CNS molecule has the overall charge of 1- so, the FC of Lewis structure has to be equal to 1-.
Resonance Hybrids
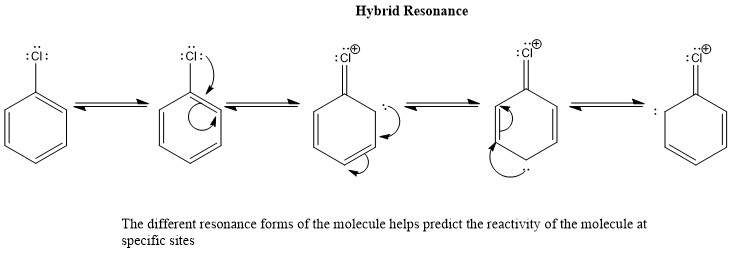
Resonance structures represent a Resonance Hybrid, which is a mixture of all resonance structures. Nearly zero (FC) Formal Charge in the resonance structure is the most acknowledged structure, nonetheless, the right Lewis structure is indeed a combination of whole resonance structures and can’t be described as only one.