When there are some changes in the system from the initial state to the final state, then it is said that the system has undergone the process. During the thermodynamic processes, the one or more properties of the system, such as the pressure, temperature, enthalpy, volume, and the heat experiences the changes. The second law of thermodynamics enables the classification of these processes under two main categories such as the ideal or reversible processes and natural or irreversible processes.
Reversible Processes
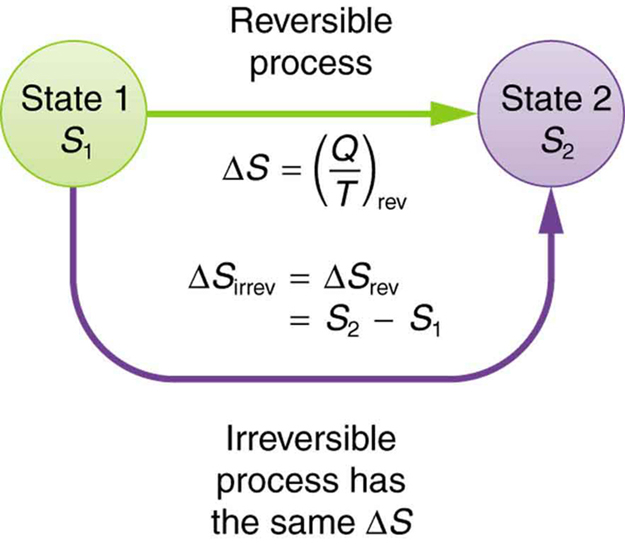
The processes in which the system and the surrounding of the system can be restored to the initial state from its final state, without causing any changes in the thermodynamic properties of the universe are known as the ideal processes or the reversible processes. For the reversible changes, the system should follow the infinitely slow rates due to the infinitesimal gradient. During the reversible processes, all of the changes that are occurring in the system are in the state of thermodynamic equilibrium with each other. The reversible processes are also termed as the isentropic processes if the heat contents of the system remain constant. The process of the systems undergoing reversible changes is also known as reversibility. In the real practices, there is no happening of the reversible processes, so it is hypothetical or the ideal process.
Irreversible Processes
The irreversible processes are also known as the natural processes as all of the processes that occur in nature are irreversible. These processes occur due to the finite gradient between two different states of the system. For example, the heat flow between two different bodies occurs due to the temperature differences between them and in fact, it is the natural flow of the heat. Similarly, the water always flows from the higher level to the lower level, and movement of the current is always from the high potential to the low potential.
Important Points About Irreversible Processes
In these processes, the initial state of the system and its surroundings cannot be restored from its final state. During these processes, various states of the system, are experiencing the path of changes from the initial to the final state, and they are not in equilibrium with each other. The entropy of the process is increased during the irreversible processes and it cannot be reduced to its original value. This overall phenomenon of the system of undergoing the irreversible process is known as the irreversibility.