Root-Mean-Square Speed
The root-mean-square speed is essential in measuring the average speed of particles contained in a gas, defined as vrms=√3RT/M.
In Kinetic Molecular Theory, gasified particles are in a condition of constant random motion; each particle moves at a completely different pace, perpetually clashing and changing directions consistently velocity is used to describe the movement of gas particles, thereby taking into account both speed and direction.
Although the velocity of gaseous particles is constantly changing, the distribution of velocities does not change. We cannot gauge the velocity of every individual particle, thus we frequently reason in terms of the particles’ average behavior. Particles moving in opposite directions have velocities of opposite signs. Since a gas’ particles are in random motion, it’s plausible that there’ll be about as several moving in one direction as within the other way, which means that the average velocity for a collection of gas particles equals zero; as this value is unhelpful, the average of velocities can be determined using an alternative method.
Root-mean square speed of molecules
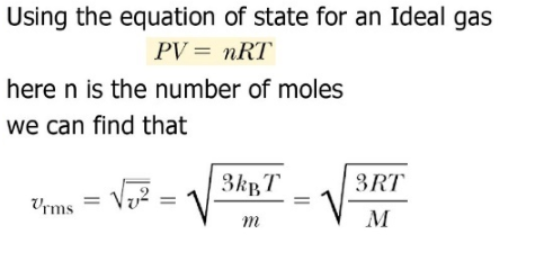
By squaring the velocities and taking the square root, we overcome the “directional” component of velocity and simultaneously acquire the particles’ average velocity. Since the value excludes the particles’ direction, we tend to currently refer to the value because of the average speed. The root-mean-square speed is used in a gas, for the measure of the speed of particles, outlined as the square root of the common velocity-squared of the molecules in a gas.It is represented by the equation: vrms=√3RT/M, wherever vrms is the root-mean-square of the speed, Mm is the molar mass of the gas in kilograms per mole, R is the molar gas constant where T is the temperature which kelvin contain.The root-mean-square speed takes into consideration both molecular weight and temperature, two factors that directly affect the kinetic energy of a material.
Root-Mean-Square Velocities of Gaseous Particles:
Measuring the velocities of particles at a given time ends up in a large distribution of values; some particles might move very slowly, others very quickly, and because they are constantly moving in completely different directions, the velocity could equal zero. (Velocity is a vector quantity, equal to the speed and direction of a particle) To properly assess the average velocity, average the squares of the velocities and take the square root of that value. This is known as the root-mean-square (RMS) velocity, and it is represented as follows:
¯v=vrms=√3RTMm
KE=12mv2
KE=12mv2
In the above formula, R is that the universal gas constant, T is absolute temperature, and millimeter is the molar mass of the gas particles in kg/mol.