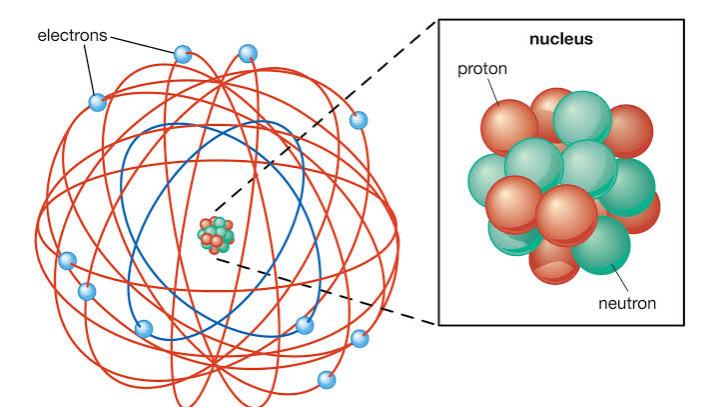
This experiment is to describe an atom he proposed that an atom is composed of empty space mostly consisting of electrons orbiting in the set around the positively charged nucleus. This comes into the consciousness of everyone after the observations made by Rutherford and those observations are that the major space in an atom is empty and the positive charge in an atom is somehow not distributed uniformly and it is concentrated in a very small volume. His model served to concentrate a great deal of the atoms charged in this another suggestion made by Rutherford that the central charge of an atom is proportional to its atomic mass. It also indicates that the atom’s electron cloud does not influence by alpha particle scattering. In most of cases atom’s positive charge is concentrated in a tiny volume at the center of an atom.
What is Rutherford’s contribution in modern science?
After Rutherford’s contribution in the atoms world scientist start learning that atom is not ultimately a single particle but made up of smaller subatomic particles. Another contribution it made is to help scientist to found the expected number of electrons in an atom by using X-rays when X-ray passes through an atom and found that some of it is scattered on the other hand some of them passes through the atom and when the X-ray loses its intensity due to scattering at electrons by noting the rate of decrease in X-ray and by this the number of electrons which are in electron can be estimated accurately. It also includes Rutherford’s gold foil experiment which includes the subsequent research which determines the exact atomic structure.
What is the difference between Thomson and Rutherford’s model?
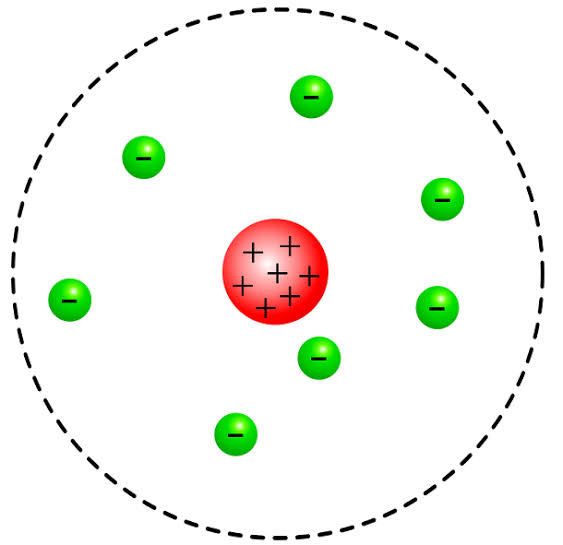
Both of them worked in the same field where they observe and conclude their observations with suitable reasons and explaining their models too. One of them proposed that an atom is composed of particles that are charged positively and the majority of the mass of an atom was concentrated in a very small region and that region is called the nucleus of the atom. The atoms of nucleus is surrounded by electrons which are negatively charged items and they revolve around the nucleus at a very high speed but at a fixed circular path. Also, atoms are electrically neutral as electrons are negatively charged and the concentrated nucleus is positively charged.