The classification of the alcohols into primary, secondary, and tertiary is done according to the position of carbon atom on which an alkyl group is attached to the hydroxyl group. Generally, these alcohols are categorized due to the presence of bent shaped hydroxyl group. For the determination of alcohols that whether it is secondary or tertiary examine the carbon that is attached to the hydroxyl group. If that carbon is attached to the two then it is secondary alcohol, and if it is attached to the three then it is tertiary alcohol.
Secondary Alcohols
In the secondary alcohols, the carbon atom of the hydroxyl group is being attached to the two alkyl groups on either side. These two alkyl groups present may be structurally different or identical. In organic chemistry oxidation of secondary alcohol to the ketone is an important oxidation reaction. The oxidation of secondary alcohols without breaking the carbon-carbon bonds is easy but up to the ketone stage only. Except for very stringent conditions, no further oxidation is observed. The acidity of secondary alcohols is between the primary and tertiary alcohols. The boiling points of secondary alcohols are also between the secondary and tertiary alcohols.
Tertiary Alcohols
In the tertiary alcohols, the hydroxyl group is attached to the carbon which is located on the third alkyl group. Mainly the physical properties are dependent on their structure. Due to the presence of this hydroxyl group in the alcohols, they are allowed to form the hydrogen bonds with their neighboring atoms. These bonds cause the higher boiling points of the alcohols than their corresponding alkanes. Without breaking the carbon-carbon bonds the oxidation of tertiary alcohols is not possible. Under the mild conditions, tertiary alcohols do not react with chromic acid.
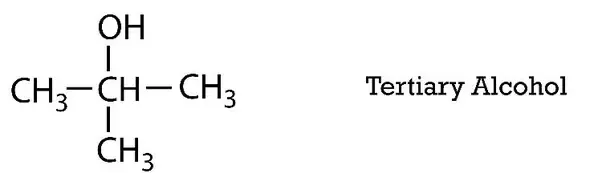
Carbon-carbon bonds can be oxidized at the high temperature or by increasing the concentration of the acid. However, from the strong oxidations, the yields are usually poor. Tertiary alcohols are the least acidic and their boiling points are also low as compare to the primary and secondary alcohols. These alcohols are widely used as beverages, as anti-freezing agents, as antiseptic, and some of them are being used as fuels in the internal combustion engines. They are also used for the preservation of the specimens in scientific experiments. They have the ability to dissolve both polar and nonpolar solvents so they are used as solvents and reagents in the industry.